Photocatalytic intermolecular anti-Markovnikov hydroamination of unactivated alkenes with N-hydroxyphthalimide
Intermolecular olefin hydroamination is an efficient strategy to form C-N bonds and then construct high-value amines. Traditional olefin hydroamination methods mainly provide Markovnikov products. Thus, it is interesting and challenging to realize anti-Markovnikov olefin hydroamination.
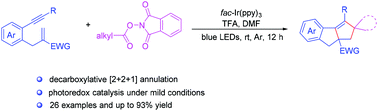
In 2019, Yang’s group reported a visible-light-induced strategy to achieve N-OH bond cleavage of strained cyclobutanone oxime, which is able to activate the N-O bond directly to construct various cyano/gemdifluoroalkene-containing scaffolds via synergistic effects between visible light and phosphoranyl radical cation (Org. Lett. 2019, 21, 2658-2662). Recently, this group designed a visible-light photoredox-catalysed hydroamination of unactivated alkenes using N-hydroxyphthalimide (NHPI) to generate anti-Markovnikov product exclusively based on previous work (Scheme 1). The high versatility and mild conditions of this strategy allow a facile access to various amines using cheap and easily available reagents.

Scheme 1 Photocatalytic intermolecular anti-Markovnikov hydroamination of unactivated alkenes with N-hydroxyphthalimide
Cyclohexene 1a and NHPI were chosen as model substrates. Optimization of reaction conditions shows that the highest yield (82%) could be obtained when using [Ir(dFCF3ppy)2dtbbpy]PF6 and P(OEt)3 as catalyst and MeCN as solvent at room temperature in 24 hours. A wide variety of unactivated alkenes could be tolerated, including cyclic and acyclic aliphatic olefins. Moreover, aliphatic olefins substituted with halogen or trimethylsilyl group could also provide the corresponding products in moderate yields (Table 2).
Table 2 Substrate Scope of Unactivated Olefinsa
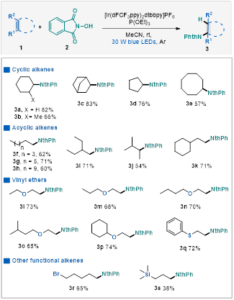
aConditions: olefin 1 (0.6 mmol, 3.0 equiv.), N‑hydroxyphthalimide (0.2 mmol, 1.0 equiv.), P(OEt)3(0.3 mmol, 1.5 equiv.), [Ir(dFCF3ppy)2dtbbpy]PF6 (2 mol%), MeCN (4 mL), 30 w blue LED, rt, argon atmosphere, 24 h, isolated yield.
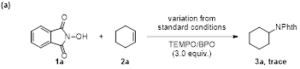
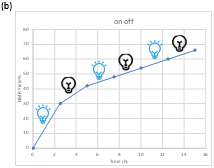
Scheme 2. Control experiments
To gain mechanistic insights into this reaction, several control experiments were then carried out. Product formation was inhibited, when radical trapping agents such as 2,2,6,6-tetramethyl-1-piperdinyloxy (TEMPO) or benzoyl peroxide(BPO) were added under the standard reaction conditions, suggesting a radical process was involved possibly. On/off experiments demonstrate the corresponding product is formed upon irradiation, as well as in the dark, which supports a chain-propagation-type radical reaction (Scheme 2).
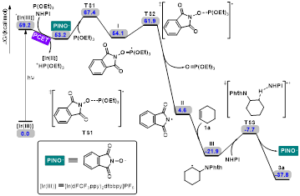
Scheme 3. potential energy surface of the plausible reaction pathway.
Furthermore, DFT calculations were performed to understand the catalytic mechanism.Initially, the [Ir(III)]isexcited to *[Ir(III)] under the irradiation of visible light. Then, NHPI and P(OEt)3are able to oxidatively quench *[Ir(III)] via a PCET-mediated activation of O-H bond to afford the corresponding PINO radical. The key PINO radical undergoes a radical addition with P(OEt)3 to deliver phosphoranyl radical I via TS1. Subsequently, N-centred radical intermediate II is formed through β-scission fragmentation of radical I, releasing triethyl phosphate. This step is calculated to possess a Gibbs free energy barrier of 7.8 kcal/mol and is highly irreversible with a driving force as large as 49.5 kcal/mol. In the presence of alkene, a radical addition occurs facilely to generate radical intermediate III. Finally, this resulting radical intermediate III promotes a hydrogen atom transfer from NHPI to deliver the desired product 3a and simultaneously regenerate the PINO radical, which would react with P(OEt)3 to initiate another radical reaction. Notably, this chain-propagation-type mechanism agrees well with our control experiments and Schmidt’s results.
In summary, this group successfully developed a visible-light photoredox-catalysed hydroamination of alkenes using N-hydroxyphthalimide (NHPI) with exclusive anti-Markovnikov selectivity. High synthetic efficiency and mild reaction conditions would endow this protocol with potentials and flexibility in building various aliphatic amines.
Corresponding author

Professor Hua Yang at College of Chemistry and Chemical Engineering Central South University has a great interest in organic synthesis, asymmetric catalysis, visible-light catalysis and total synthesis of chiral drug molecules. Professor Yang developed an excellent organic catalyst-“Hua Cat”. And this patented reagent was commercialized by Sigma-Aldrich, and has been widely applied in asymmetric synthesis.

Hao-Yue Xiang, is an associate professor at College of Chemistry and Chemical Engineering, Central South University. Dr. Xiang received his PhD degree from Shanghai Institute of Materia Medica, Chinese Academy of Sciences and has made great progresses in the construction of heterocyclic compound library and the discovery of lead compounds. Dr Xiang’s current research focus is on fluorine chemistry, boron chemistry and radical chemistry.

Dr Kai Chen, at College of Chemistry and Chemical Engineering, Central South University.mainly work in computational organic chemistry, designof autocatalytic system, and computer-aided drug design. Chen received his PhD at Peking University in 2014, and then moved to South China University of Technology. Since 2019, Chen worked at Central South University.

Peng-Ju Xia, is lecturer of School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University.Xia mainly work inresearch field ofphotocatalytic/electrochemical organic catalysis and and 1, 3-dipole cyclization.
Comments Off on Article Highlight: Photocatalytic intermolecular anti-Markovnikov hydroamination of unactivated alkenes with N-hydroxyphthalimide