Organic Chemistry Frontiers is delighted to share with you the HOT articles of May 2018!
You can access these publications for free till 31th July 2018 by logging into your free Royal Society of Chemistry publishing personal account (http://pubs.rsc.org).
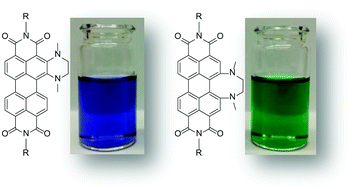
Efficient catalytic vicinal diamination of arylene diimides
Michael Kremer, Maximilian Kersten and Sigurd Höger
Org. Chem. Front., 2018,5, 1825-1829
http://dx.doi.org/10.1039/C8QO00222C
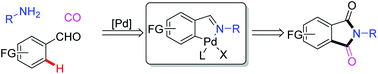
Azidoheteroarylation of unactivated olefins through distal heteroaryl migration
Min Wang, Zhen Wu, Bo Zhang and Chen Zhu
Org. Chem. Front., 2018, Advance Article
http://dx.doi.org/10.1039/C8QO00301G
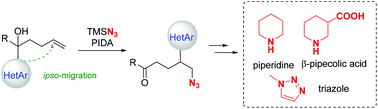
Selective formation of phthalimides from amines, aldehydes and CO by Pd-catalyzed oxidative C–H aminocarbonylation
Renyi Shi, Fan Liao, Huiying Niu and Aiwen Lei
Org. Chem. Front., 2018, Advance Article
http://dx.doi.org/10.1039/C8QO00282G
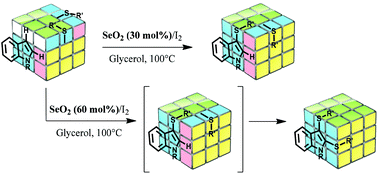
Selenium dioxide-promoted selective synthesis of mono- and bis-sulfenylindoles
Samuel Thurow, Filipe Penteado, Gelson Perin, Diego Alves, Claudio Santi, Bonifacio Monti, Carl H. Schiesser, Thiago Barcellos and Eder J. Lenardão
Org. Chem. Front., 2018, Advance Article
http://dx.doi.org/10.1039/C8QO00360B
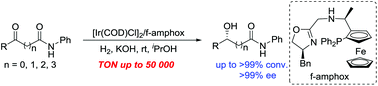
Highly enantioselective Ir/f-amphox-catalyzed hydrogenation of ketoamides: efficient access to chiral hydroxy amides
Yang Hu, Xuguang Yin, Ziyi Chen, Xiu-Qin Dong and Xumu Zhang
Org. Chem. Front., 2018, Advance Article
http://dx.doi.org/10.1039/C8QO00307F
A more reliable synthesis of a Gemini vitamin D analog, a potentially effective chemotherapeutic agent for the treatment of colorectal carcinomas
Hugo Santalla, Fátima Garrido, Generosa Gómez and Yagamare Fall
Org. Chem. Front., 2018, Advance Article
http://dx.doi.org/10.1039/C8QO00338F

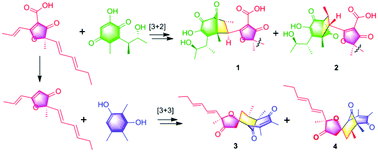
Three-component reaction to synthesize E-vinyl silyl anti-1,2-diols via sequential [1,4]-O-to-O/[1,4]-C-to-O silyl migrations
Qiang Pu, Xiaoxiao Tang, Lu Gao and Zhenlei Song
Org. Chem. Front., 2018, Advance Article
http://dx.doi.org/10.1039/C8QO00332G
![Graphical abstract: Three-component reaction to synthesize E-vinyl silyl anti-1,2-diols via sequential [1,4]-O-to-O/[1,4]-C-to-O silyl migrations](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00332G)
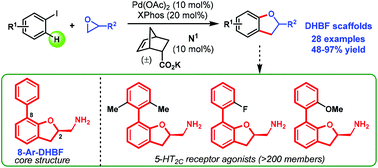
Convergent syntheses of 2,3-dihydrobenzofurans via a Catellani strategy
Chenggui Wu, Hong-Gang Cheng, Ruiming Chen, Han Chen, Ze-Shui Liu, Jingyang Zhang, Yuming Zhang, Yuxin Zhu, Zhi Geng and Qianghui Zhou
Org. Chem. Front., 2018, Advance Article
http://dx.doi.org/10.1039/C8QO00348C
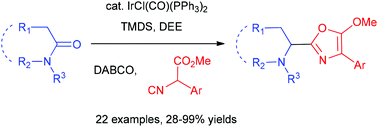
The iridium-catalysed reductive coupling reaction of tertiary lactams/amides with isocyanoacetates
Xiu-Ning Hu, Tai-Long Shen, Dong-Cheng Cai, Jian-Feng Zheng and Pei-Qiang Huang
Org. Chem. Front., 2018, Advance Article
http://dx.doi.org/10.1039/C8QO00312B
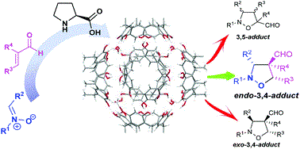
Asymmetric [4 + 2] cycloadditions with 3-furfural derivatives and α-cyano-α,β-unsaturated ketones
Chuan-Qi Duan, Xiao-Long He, Wei Du and Ying-Chun Chen
Org. Chem. Front., 2018, Advance Article
http://dx.doi.org/10.1039/C8QO00351C
![Graphical abstract: Asymmetric [4 + 2] cycloadditions with 3-furfural derivatives and α-cyano-α,β-unsaturated ketones](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00351C)













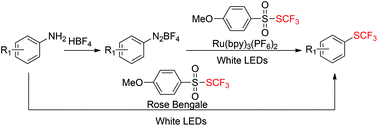
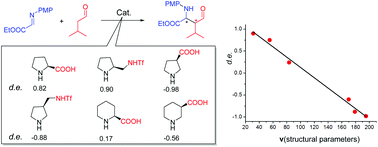
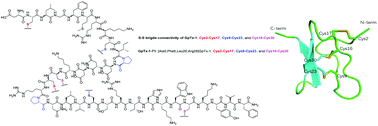

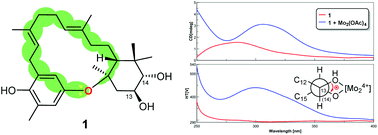
![Graphical abstract: Access to sulfonylated furans or imidazo[1,2-a]pyridines via a metal-free three-component, domino reaction](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00443A)






![Graphical abstract: Three-component reaction to synthesize E-vinyl silyl anti-1,2-diols via sequential [1,4]-O-to-O/[1,4]-C-to-O silyl migrations](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00332G)


![Graphical abstract: Asymmetric [4 + 2] cycloadditions with 3-furfural derivatives and α-cyano-α,β-unsaturated ketones](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00351C)
![Graphical abstract: Enantioselective indium(i)-catalyzed [4 + 2] annulation of alkoxyallenes and β,γ-unsaturated α-keto esters](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00319J)
![Graphical abstract: Base-promoted [3 + 3] cyclization of cyclopropenones and cyclopropenethiones with amides for the synthesis of 6H-1,3-oxazin-6-ones and 6H-1,3-thiazin-6-ones](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00091C)
![Graphical abstract: Efficient access to chiral benzo[c]chromenes via asymmetric transfer hydrogenation of ketals](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00096D)