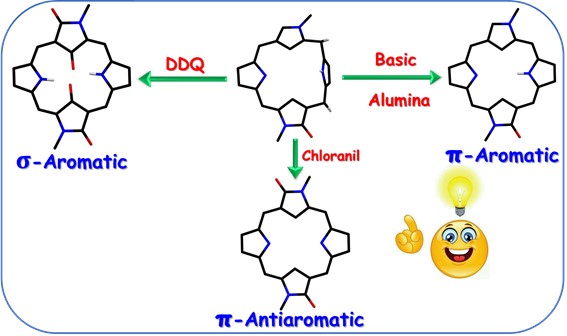
Indian scientist, Dr Harapriya Rath and her collaborator Dr. Dandamudi Usharani experimentally isolated and theoretically supported the first ever s-aromatic doubly N-confused porphyrinoid. Unique π-reconstructions owing to sequential C-oxygenation of N-confused N-methyl pyrrole rings leading to the genesis of 18π aromatic doubly N-confused monooxo porphyrinoid, 16π antiaromatic doubly N-confused diioxo porphyrinoid and σ-aromatic doubly N-confused tetraoxo isophlorinoid via chemical transformation(s) of doubly N-confused porphodimethene.
Corresponding Author:
Harapriya Rath received her PhD degree from the Indian Institute of Technology, Kanpur, India on the Nonlinear Optical studies of Core-modified Aromatic Expanded porphyrinoids under the supervision of Prof. T. K. Chandrashekar in 2006. In 2007, she joined the group of Prof. Hiroshi Shinokubo at the Kyoto University, Japan as JSPS Postdoctoral fellow and focused on the subject of Möbius aromaticity (antiaromaticity) and excited state aromaticity of metallated all-aza expanded porphyrinoids. In 2009, she moved to Prof. Richard EP Winpenny’s lab at the University of Manchester, United Kingdom as a Royal Society Newton International postdoctoral fellow focusing on hybrid Organic-Inorganic Rotaxanes. In 2012, she started an independent academic career at Indian Association for the Cultivation of Science, Kolkata, India as an Assistant professor and Ramanujan Fellow. Since 2017, she is Associate Professor in the School of Chemical Sciences, IACS, Kolkata, India.