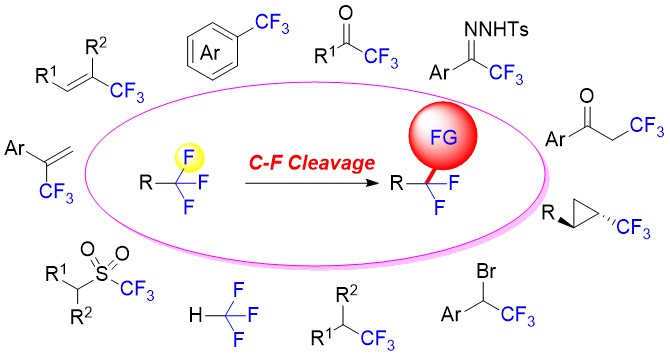
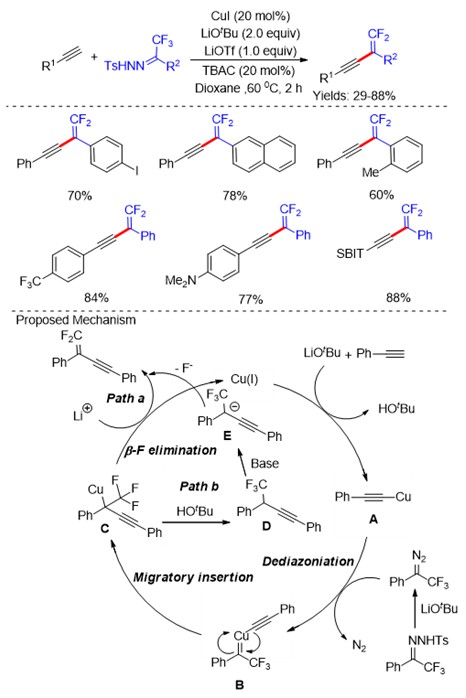
Organofluorine compounds play an important role in pharmaceuticals, agrochemicals, and functional materials, due to their special chemical, physical and biological properties, such as increased electronegativity, hydrophobicity, bioavailability and metabolic stability. Therefore, great efforts have recently been devoted to the development of new methods for the synthesis of fluorinated compounds. Conventional strategies mainly focus on the selective introduction of fluorine atom or fluorine-containing moiety into organic molecules. Alternatively, the development of novel synthetic methodologies via the selective activation of C-F bond is of vital importance, which could allow for the synthesis of partially fluorinated synthetic intermediates from readily available polyfluorinated starting materials. The research of C-F bond activation is the most challenging task in organic synthesis, which has recently drawn increasing attention from chemists. In this review, we mainly focus on the C-F bond activation of CF3 groups for the synthesis of fluorinated compounds as well as discussion of their mechanisms(Scheme 1).
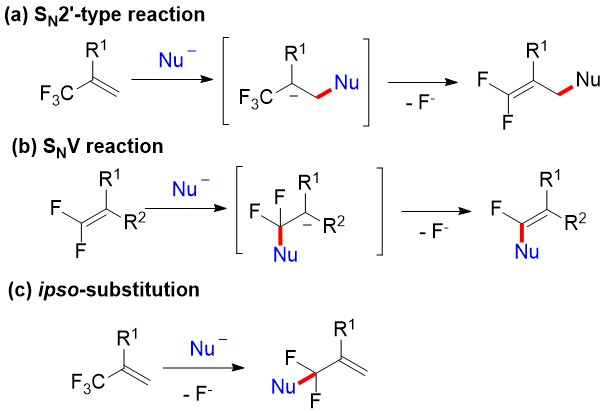
Due to the electron-deficient property, α-trifluoromethylstyrenes exhibit unique reactivities in their C-F bonds activation, mainly including three kinds of reactions (SN2′-type, SNV and ipso-substitution reactions, Scheme 2).
The cleavage of C-F bond in trifluoromethylated aromatic (Scheme 3) and alkyl compounds (Scheme 4) could be achieved through using transition metal complexes, main-group Lewis acids and base-conditions. An alternative strategy mainly relied on the single-electron transfer (SET) via Low-valent metals, irradiation with visible light.
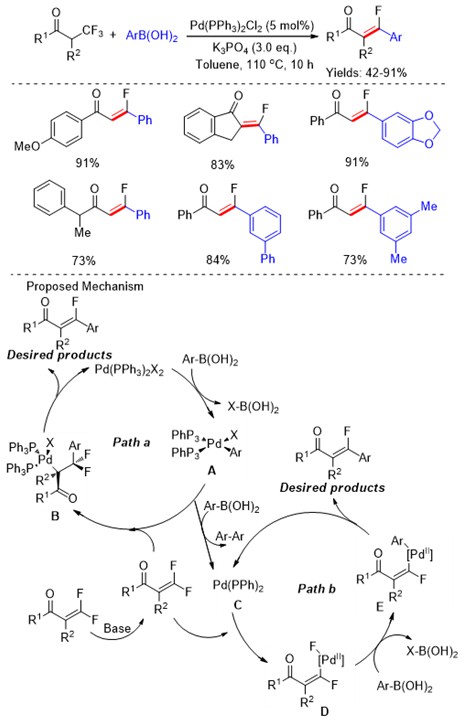
Scheme 4 Pd-catalyzed defluorination/arylation reaction of α-trifluoromethyl ketones with aryl boronic acids.
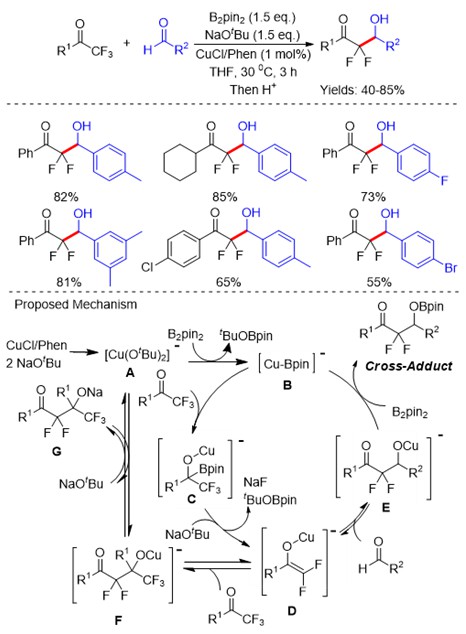
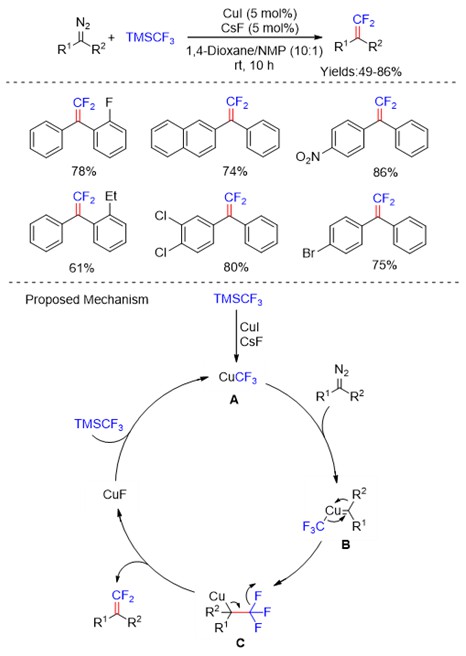
Trifluoromethyl ketones (Scheme 5) and their corresponding diazo compounds (Scheme 6) , N‑tosylhydrazones (Scheme 7) have been utilized as the coupling partners in modern synthetic organic chemistry. Recently, the C-F bond cleavage of these compounds has also been reported through transition-metal catalysts.
Although great progress has been made in this rapidly developing area, these reactions still suffer from some issues of the limitation of substrates and poor regioselectivity. To overcome these central challenges, it is more important to extend the substrate scope of the existing methodologies, especial trifluoromethylated alkyl compounds. Furthermore, the robust catalytic systems will be developed for the selective activation of a single C-F bond in CF3 groups.
Guobing Yan
Zhejiang A&F University
Guobing Yan is a Professor in college of Jiyang at Zhejiang A&F University. He obtained B.Sc. degree from Jinggangshan Normal University, his M.Sc. degree from Suzhou University, and his Ph.D. degree from Tongji University in 2010. He spent two years in 2008 and 2009 as visiting student in professor Jianbo Wang’s laboratory at Peking University. In 2013, He joined Dr. Dong’s group at the University of Texas at Austin as a visiting professor. His current research interests focus on the transition-metal-catalyzed activation of inert chemical bonds and green synthetic chemistry. He is the author of 6 patents and more than 70 articles indexed by SCI.
Recent Advance in the C-F bond functionalization of trifluoromethyl-containing compounds
Guobing Yan, Kaiying Qiu and Ming Guo