In the emerging technologies of organic electronics, positional isomerism has appeared in recent years as an efficient molecular tool to tune the electronic and physical properties of organic semi-conductors (OSCs), which are at the heart of the devices. For example, modifying the position of the linkages (ortho, meta, para), allows to extend or restrict the π-conjugation between two molecular fragments and, all the key electronic parameters of an OSC can be easily modified (HOMO / LUMO energy levels, single and triplet state energies, charge carriers mobilities…) leading to substantial different performances in electronic devices.
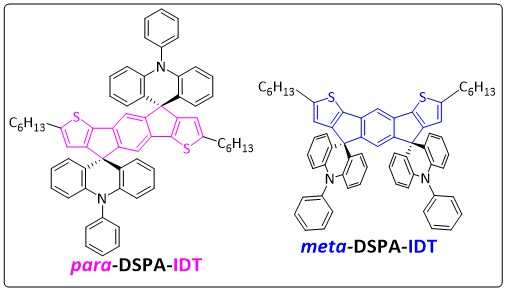
The indacenobisthiophene (IDT) fragment, which is an association of two thienyl cores to a central phenyl ring, has appeared in the last ten years as an important building unit to construct efficient organic materials mainly for organic photovoltaics but also for Organic Field-Effect Transistors (OFETs). However, with a few exceptions, nearly all the IDT-based molecules described to date in the literature are constructed on a central para-linked phenyl ring with the two thienyl sulfur atoms in an anti-configuration (see para-IDT core in pink in Chart 1). Recently, the study of IDT positional isomers possessing a central meta-linked phenyl unit with the two sulfur atoms in a syn configuration (see meta-IDT core in blue in Chart 1) has allowed to show the impact of positional isomerism on this family of compounds.
Continuing this systematic approach, the authors investigate herein the incorporation of the widely known electron rich phenylacridine (PA) fragment spiro-linked to the meta- or para-IDT core. Thus, DiSpiroPhenylAcridine-IDT isomers, para-DSPA-IDT and meta-DSPA-IDT, constructed on the so-called “3π-2spiro” architecture, have been investigated through a detailed structure-properties relationship study.
In the present work, the group of Prof Cyril Poriel and Prof Joëlle Rault-Berthelot (Institut des Sciences Chimiques de Rennes- UMR 6226, Rennes) reports that the phenylacridine fragment significantly modifies the electronic, physical and charge transport properties compared to structurally related DiSpiroFluorene-IDT analogues (para-DSF-IDT, meta-DSF-IDT). This finding is different to what was reported in literature for other couples of IDT-based isomers and shows the key role played by the spiro-connected fragments on the charge transport properties of these molecular systems and open new avenues for meta-substituted oligomers.
Despite that the properties are mainly driven by the IDT core, this work shows that the bridges (herein spiro-connected phenylacridine) allow a fine tuning of all the properties. Particularly, the electrochemical studies have revealed the strong differences observed in the oxidation of the two isomers.
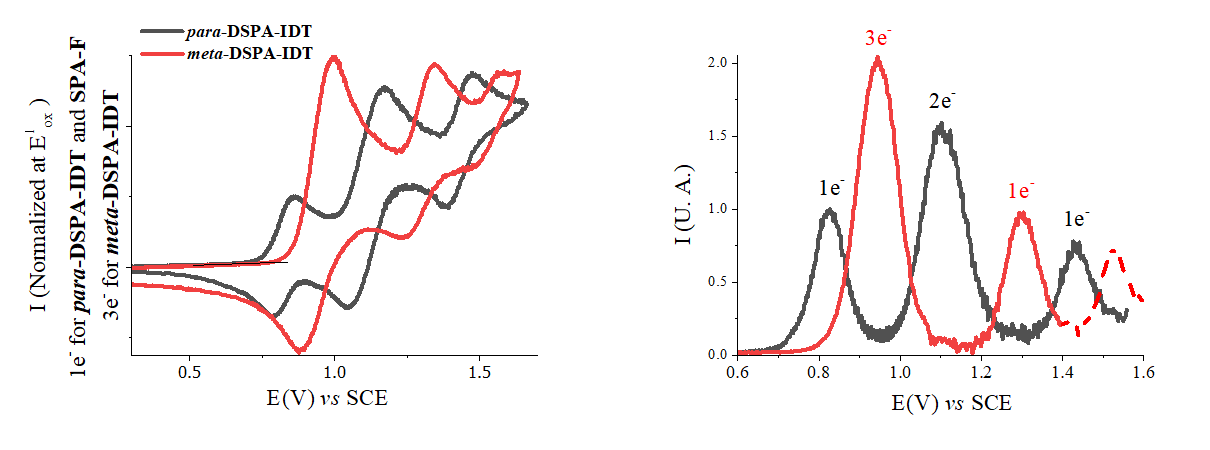
Figure 1. Left, CVs of para-DSPA-IDT (1.5 × 10-3 M) and of meta-DSPA-IDT (2.15 × 10-3 M) recorded between 0.25 and 1.8 V, sweep-rate 100 mV.s-1. Right: DPVs normalized on the first oxidation wave for para-DSPA-IDT and on the second oxidation wave for meta-DSPA-IDT. Pulse Height: 25 mV, sweep-rate 50 mV.s-1, t: 50 ms. Working platinum disk electrode (Ø 1 mm).
This work also shows that the mobilities of charge carriers are higher for the meta isomer than for the para isomer. This finding is different to what was reported in literature for other couples of IDT-based isomers and shows the key role played by the spiro-connected fragments on the charge transport properties of these molecular systems.
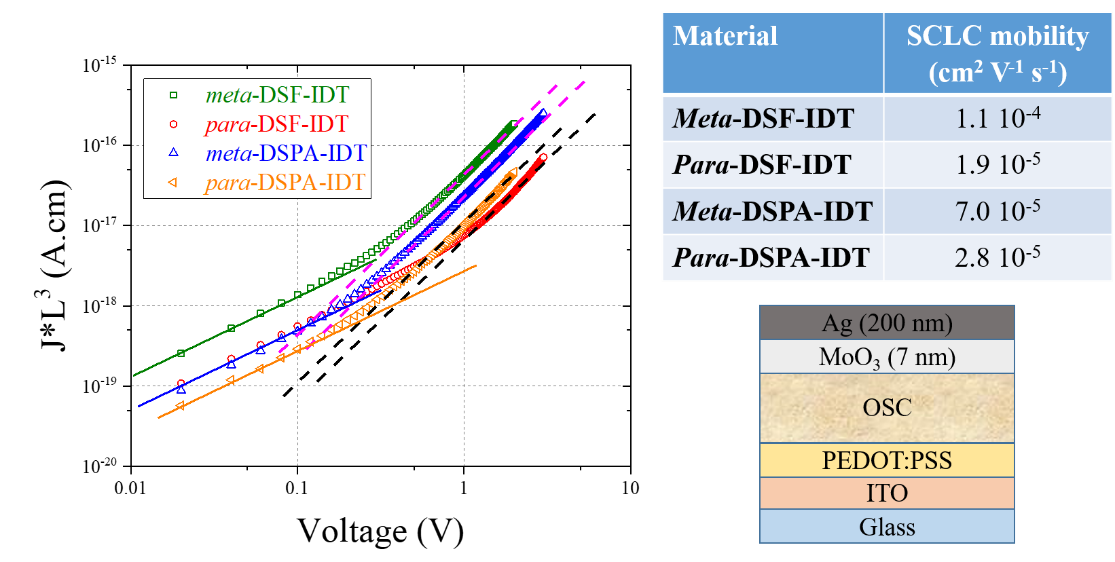
Figure 2. Thickness-scaled current-voltage characteristic of the para– and meta-DSPA-IDT and para– and meta-DSF-IDT hole-only SCLC devices (Left), SCLC mobility and SCLC device (right). In left, the dotted lines indicate the SCLC regime and the continuous ones the Ohmic regime.
BIOGRAPHICAL INFORMATIONS
Cyril Poriel received his PhD in 2003 from the University of Rennes 1. After a postdoctoral stay at the University of Exeter (UK), he joined the CNRS (Institut des Sciences Chimiques de Rennes) in 2005, where he is currently CNRS Research Director. His main research interest deals with the design of π-conjugated architectures for Organic Electronics. He is author/co-author of more than 120 publications, reviews and book chapters.
Joëlle Rault-Berthelot received her PhD in 1986 from the University of Rennes 1, where she is currently CNRS research director (Institut des Sciences Chimiques de Rennes). She has been working for 40 years in electrochemistry. Since 2005, she is involved in the design of π-conjugated systems for Organic Electronics and is author/co-author of more than 160 publications, reviews and book chapters.