The efficiency of water splitting is severely limited by the oxygen evolution reaction (OER), due to sluggish kinetics and a substantial overpotential. To overcome this challenge, precious-metal based catalysts, such as IrO2 and RuO2, have been investigated and confirmed to exhibit good OER performance. However, the scarcity and high cost of these materials restrict their large-scale application.
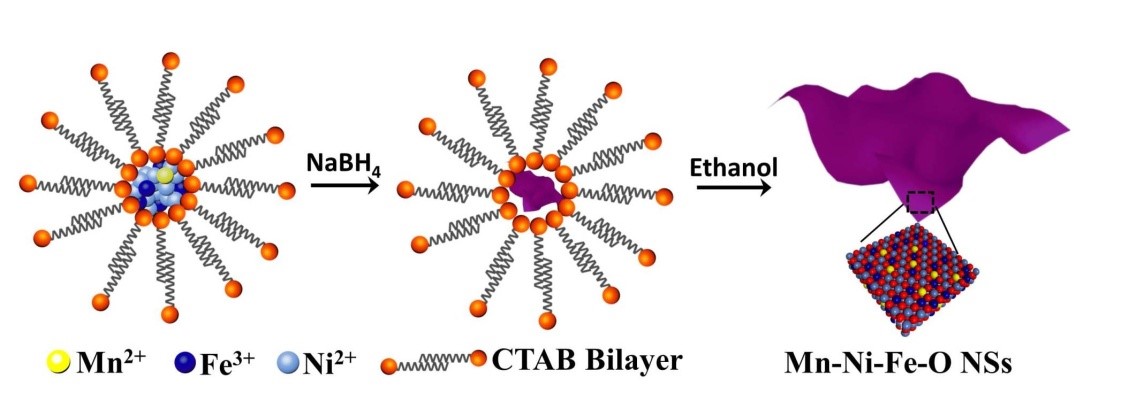
Recently, the group of Derek Ho and collaborators of the City University of Hong Kong have demonstrated a one-step method for the synthesis of Mn doped ultrathin nickel-iron oxide (Mn-Ni-Fe-O) nanosheets, which simultaneously achieves an abundance of oxygen vacancies and high valance Ni3+ catalytic sites (Fig. 1). The Mn dopant exists in the form of mixed-valence Mn cations, which contributes to tailoring the electronic structure of the Ni and Fe sites, leading to outstanding OER catalytic performance.
Figure 1. Schematic of the preparation procedure of Mn-Ni-Fe-O nanosheets.
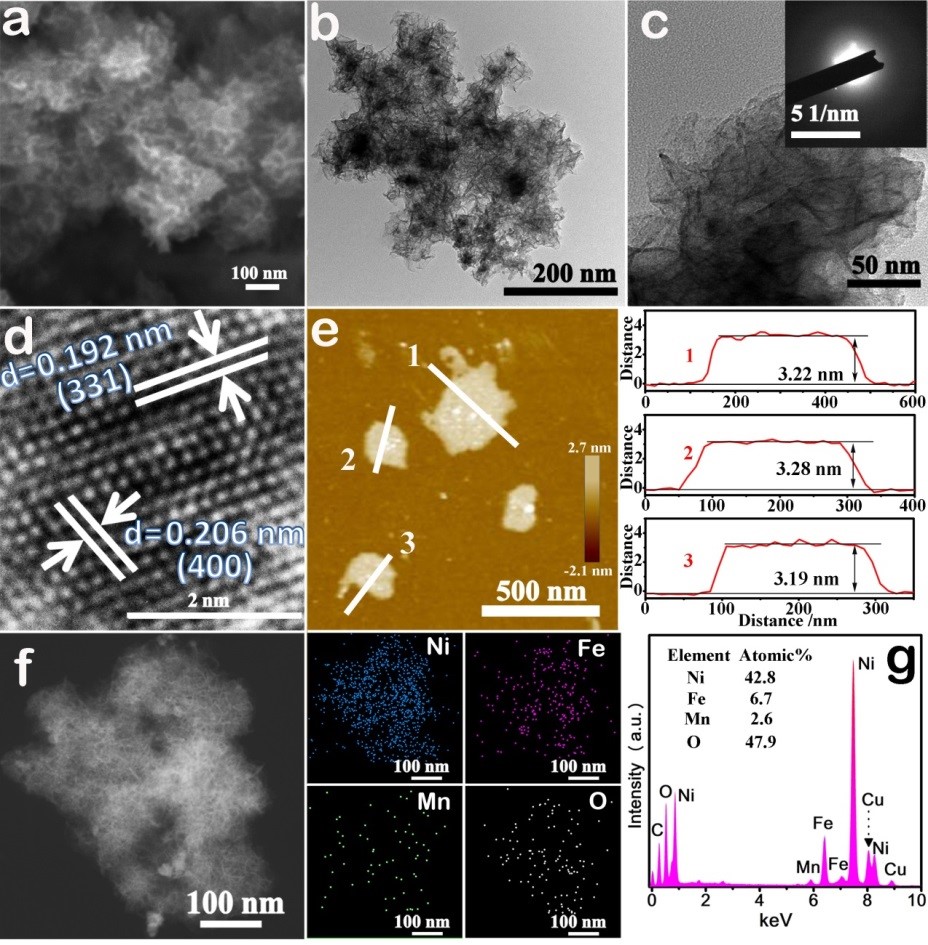
SEM and TEM images of the Mn-Ni-Fe-O hybrid shows 100 – 300 nm interconnected nanosheet structures, having an ultrathin and veil-like morphology (Fig. 2). AFM images show a nanosheet thickness of approximately 3.2 nm. EDX mapping presents that Ni, Fe, Mn, and O elements are uniformly dispersed throughout the nanosheets.
Figure 2. (a) SEM image, (b, c) TEM images and the inset in (c) is the corresponding SAED patterns, (d) HRTEM image, (e) AFM image and the corresponding thickness curve, (f) STEM image and the corresponding element mapping, and (g) EDX spectrum of the Mn-Ni-Fe-O nanosheets.
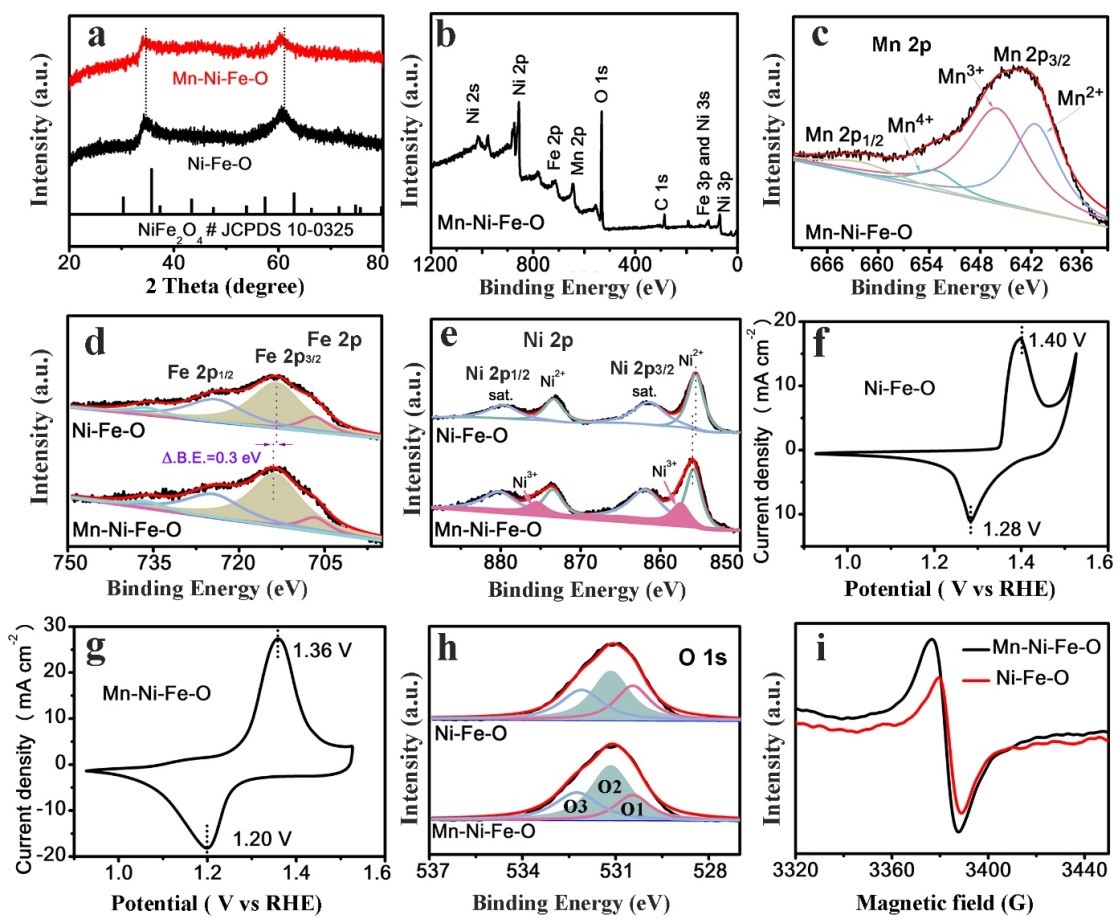
XRD, XPS, CV, and EPR are also performed (Fig. 3). From XPS, after Mn doping, the Fe 2p3/2 XPS peak of the Mn-Ni-Fe-O nanosheets shifts to a higher binding energy as compared to that of undoped Ni-Fe-O nanosheets, suggesting that Mn dopant can modulate the charge density of Fe atom sites. Compared to the Ni 2p XPS spectrum of pristine Ni-Fe-O, the Ni 2p XPS spectrum of Mn-Ni-Fe-O nanosheets exhibits an obvious positive shift of 0.3 eV in binding energy, which is attributed to Mn incorporation. From CV curves, the Ni2+ oxidation peaks appear at 1.40 and 1.36 V versus RHE for the undoped and doped samples, respectively, indicating the oxidation of Ni species is enhanced upon Mn doping. Also, the O2 ratio (51.0 %) for the Mn-Ni-Fe-O nanosheets is higher than that of the Ni-Fe-O nanosheets (41.9 %), which indicates that Mn dopants can create an enhanced oxygen vacancies concentration.
Figure 3. Characterization data of the Mn-Ni-Fe-O and Ni-Fe-O: (a) XRD patterns, (b) XPS survey spectra, (c) high-resolution XPS spectra for Mn 2p region for Mn-Ni-Fe-O, (d) XPS for the Fe 2p region, (e) XPS for the Ni 2p region, CV curves (scan rate of 50 mV s-1) of (f) Ni-Fe-O and (g) Mn-Ni-Fe-O, (h) XPS for the O 1s region, and (i) electron paramagnetic resonance (EPR) spectra of Mn-Ni-Fe-O (2 wt%).
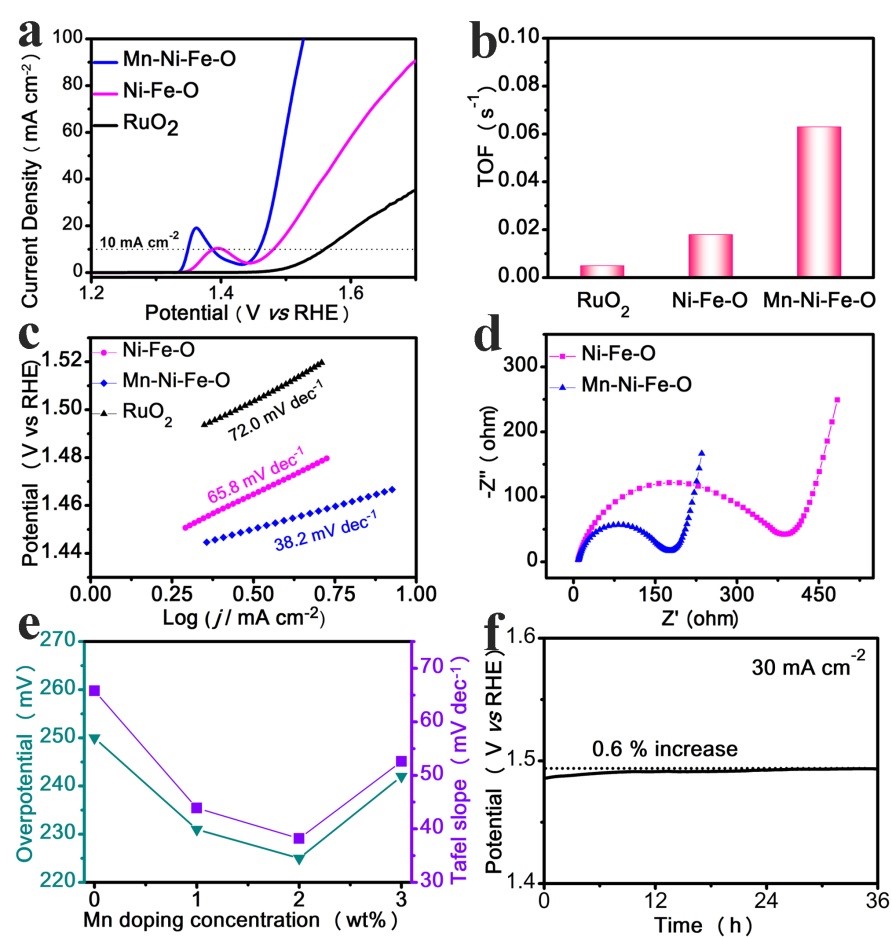
OER electrochemical performance has been investigated in an O2-saturated KOH (1 M) solution. Upon doping of Mn, polarization curves show an OER overpotential of only 225 mV (vs. undoped at 250 mV) (Fig. 4). Remarkably, these two as-prepared ultrathin nanosheets, with or without Mn doping, exhibit faster OER than the commercial RuO2. The Mn doped nanosheets exhibit a turnover frequency (TOF) of 0.063 s−1 at the overpotential of 300 mV, which is 3.5 and 12 times higher than that of the undoped sample and commercial RuO2, respectively. The Tafel slope is 38.2 mV dec-1 (vs. 65.8 mV dec-1 undoped and 72.0 mV dec-1 from RuO2). Electrochemical impedance spectroscopy (EIS) reveals that the Mn dopants can effectively improve the electrical conductivity.
Figure 4. (a) Polarization curves, (b) TOF, and (c) Tafel slope of Mn-Ni-Fe-O, Ni-Fe-O, and RuO2. (d) Nyquist slopes of Ni-Fe-O and Mn-Ni-Fe-O, (e) overpotential at 10 mA cm-2 and Tafel slope of Ni-Fe-O nanosheets with different Mn doping levels, and (f) chronopotentiometry curves of Mn-Ni-Fe-O nanosheets at 30 mA cm-2.
This work demonstrated a facile method in synthesizing ultrathin Mn-Ni-Fe-O nanosheets that achieve highly efficient OER catalytic performance, providing a sound strategy for the design and synthesis of multi-metallic, atomically-thin oxides nanosheets to mitigate the catalytic limitation of OER, thereby rendering the electrolysis of water a practical form of alternative fuel production.
Information on Corresponding Author
Derek Ho
City University of Hong Kong
Derek Ho is currently an associate professor at the Department of Materials Science and Engineering at City University of Hong Kong. He directs the Atoms to Systems Laboratory. He received his B.A.Sc. (first class) and M.A.Sc. in Electrical and Computer Engineering from the University of British Columbia (UBC), Vancouver, Canada, in 2005 and 2007 respectively. At UBC, he focused his study on microelectronics. He received his Ph.D. in Electrical and Computer Engineering from the University of Toronto, Toronto, Canada in 2013, where he worked on sensors incorporating nanomaterials and CMOS electronics for chemical detection and DNA biosensing applications. Professor Ho’s research interest is in the synthesis of electronic nanomaterials and fabrication of advanced devices. His current research focuses on sensing and energy applications, mainly in the form of stretchable and healable electronics. www.atomstosystems.com
Article information:
Mn dopant induced high-valence Ni3+ sites and oxygen vacancies for enhanced water oxidation
Yu Zhang, Zhiyuan Zeng and Derek Ho
Mater. Chem. Front., 2020, Advance Article