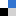Since the early study at the beginning of the XX century of the first macrocycles ever analysed, among them the azacycloalkane 1,4,8,11-tetraazacyclotetradecana -more commonly known as cyclam-, such cyclic structures have shown compelling and encouraging properties. Indeed, beyond their broad use in nature –including roles both as signalling molecules and metabolites employed in modulating immune-system responses-, synthetic macrocycles have been used, for instance, to facilitate ion transport across hydrophobic membranes, drug delivery and the development of molecular machines.
Among these compounds, aza-macrocycles –and particularly those that include a pyridine ring in their structure, such as those showed in Figure 1- generally allow for strong coordination of metal cations which anyhow leaves free positions in the cations’ coordination sphere; this is an engaging feature to take advantage of. Thus, in last decades, a set of design strategies have been developed for exploiting these features in catalysis, recognition, enzyme mimicking and inhibition, interactions with macromolecules, and so forth. Among them we recall the main, perhaps first-generation, approach, of converting the macrocycle into the head of a scorpiand-type ligand featuring a functional tail with either further donor atoms, binding sites for supramolecular guests, adequate groups for grafting on surfaces, etc.
Recently, the groups of Antonio Bianchi (University of Florence) and Enrique García-España (University of Valencia) have focused on the effect of small structural changes (methylation) in the ability of aza-macrocycle-based Cu(II) complexes of stabilizing polyiodide networks, as a controlled manner to simultaneously boost electronic properties and robustness of these materials.
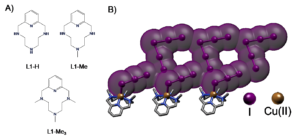
Figure 1. A) Structure of the three analogous polyazacyclophanes studied by the groups of professors Bianchi and García-España. B) Representation of a polyiodide network stabilized by the Cu(II) complexes of the azamacrocyclic ligands playing a counterion role.
The groups have demonstrated how the three analogous polyazacyclophanes shown in Figure 1 form the same CuL2+ complex as prevalent species in solution, so that a level playing field exist where only N‑methylation distinguishes them. Then they used them as countercation for polyiodide growth.
XRD analysis on the resulting crystals clearly show that methylation is a valuable tool to gradually shift directional control of subtending pairing preferences from H-bond to I···I interactions: this affects global packing and actively incorporates metal centres into polyiodide chains, setting the scene for further developments.
Authors:
Matteo Savastano
University of Florence
Álvaro Martínez-Camarena
University of Valencia
Article information:
Stabilization of polyiodide networks with Cu(II)-complexes of small methylated polyazacyclophanes: shifting directional control from H-bonds to I∙∙∙I interactions
Álvaro Martínez-Camarena, Matteo Savastano, Jose Miguel Llinares, Begoña Verdejo, Antonio Bianchi, Enrique García-España and Carla Bazzicalupi
Inorg. Chem. Front., 2020, Accepted Manuscript
https://doi.org/10.1039/D0QI00912A