The development of carbon neutral, or better still carbon zero (H2) future fuels with production driven by green energy (renewables, such as solar, wind or wave generated energy) is an urgent necessity. To be cost competitive, green production of hydrogen requires long lived, high activity catalysts made from inexpensive, earth abundant metal ions.
Over the last few decades, various earth-abundant molecular cobalt, iron and nickel catalysts have exhibited activity for HER under photo- and electro-catalytic conditions. Prior to this study only 15 molecular copper complexes had been tested as catalysts for the H2 evolving reaction (HER) under either photo or electro-catalytic conditions.
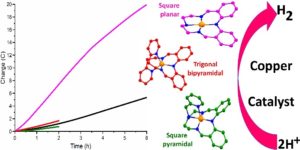
Recently, Abdullah Abudayyeh in the group of Sally Brooker at the University of Otago and collaborators Garry Hanan and Olivier Scott at the University of Montreal reported the results of testing three copper(II) complexes – with a range of geometries, from square planar 1 to square pyramidal 2 and trigonal bipyramidal 3 (Figure 1) – as catalysts for HER under both photo- and electro-catalytic conditions.
Figure 1. Right: Square planar 1 (pink), square pyramidal 2 (red) and trigonal bipyramidal 3 (green) copper(II) complexes. Left: HER electrocatalysis data shows that only square planar([CuIILEt]BF4 is, or forms, a good electrocatalyst for HER, and that it is still active after 6 hours. Black line = control.
The research team showed that under photocatalytic conditions the 3 copper complexes have modest turnover numbers (TON=460-620), but the control, using Cu(BF4)2, had a higher TON (740), and the blank (no copper) also had significant activity (TONequiv=290). So this is a cautionary tale: whilst these complexes initially appeared to be promising catalysts for photocatalytic HER, running the control and blank – studies often not reported – shows otherwise.
Hence the team changed focus and tested all three copper complexes as HER electrocatalysts in MeCN/acetic acid solution (Figure 1). The macrocyclic square planar complex, [CuIILEt]BF4 (1) (Figure 1, pink), is shown to be, or to form, an effective and stable electrocatalyst for HER in MeCN with acetic acid as the proton source (TON = 12.5 over six hours at -1.6 V vs 0.01 M AgNO3/Ag; at 100 mVs-1, Ecat/2=-1.64 V so overpotential necessary for catalysis=0.23 V), whilst the other two complexes, 2 and 3, had activities similar that of the control.
Preliminary ‘rinse and repeat’ and ‘drop of Hg’ tests – for the formation of catalytically active heterogeneous deposit on the glassy carbon working electrode or nanoparticles, respectively – are consistent with homogeneous catalysis by 1. But the authors note that it is distinctly possible that these initial test results are ‘false negatives’ (they recommend reading the excellent review by J. Dempsey on this topic), as the CVs show a stripping process consistent with the deposition of metallic copper on the electrode, so it remains possible that a heterogeneous catalytically active species forms, but is not seen as it is unstable in air or falls off the electrode during the rinse step.
Future studies on 1 aim to determine whether the catalytically active species is homogeneous or heterogeneous, but regardless of the outcome, the observed HER performance is promising and long lived.
Left to right: Abdullah, Olivier, Humphrey, Garry and Sally
Abdullah M. Abudayyeh completed his BSc in Chemistry in Mu’tah University, Jordan. He received his MSc in organic chemistry from the University of Jordan, working on Schiff base amidine systems. On successful completion of his MSc he worked as a chemistry teacher at the United Nations secondary school in Jordan for five years and then in a couple of other schools in Jordan, before moving to the University of Bradford, UK, in 2015. There he pursued his second MSc degree, developing thin films of Zr-based metal-organic frameworks (MOFs) on a conductive FTO substrate, which he completed with distinction. He then moved to Dunedin, New Zealand, to take up a University of Otago PhD scholarship in Professor Sally Brooker’s research group, working on coordination complexes as catalysts for the hydrogen evolution reaction (HER).
Olivier Schott graduated with the Bachelor of Chemistry and two Masters: Supramolecular and Molecular Chemistry and Chemical Physics and Materials at Université de Strasbourg (France). His first steps in research with Professor Julve and Professor Kurmoo were related to the synthesis of supramolecular inorganic architectures for the study of magnetic exchange interactions between transition metals. During an international student exchange, he joined the Green Energy Group of Université de Montreal (Canada) under the direction of Professor Garry S. Hanan and became a PhD candidate. In the domain of molecular artificial photosynthesis, he is investigating various poly-metallic N-rich supramolecular systems for the photocatalysis of solar fuels. In 2019, he got a PhD fellowship: Fonds Québécois de la Recherche sur la Nature et les Technologies (FRQNT) and received three times the J. Armand Bombardier Excellence Award.
Humphrey L. C. Feltham obtained his MSc and PhD (both with distinction) in Chemistry from the University of Otago under the supervision of Professor Sally Brooker, working on tetrametallic 3d-4f macrocyclic Single Molecule Magnets. From 2012-2018 he was a research associate in Professor Brooker’s team, working on projects ranging from the tuning of spin crossover complexes to the covalent immobilisation of magnetically and catalytically interesting complexes onto Au nanoparticles and PEDOT films. In 2019, he joined the chemistry team at Ligar Limited Partnership (www.ligar.nz), developing novel polymers for the removal or recovery of valuable or unwanted molecules from a variety of solutions. eg. removing smoke taints from wine, recovering bioactive molecules from plant extracts, remediating toxins from drinking water, and modifying flavour of beverages.
Professor Garry S. Hanan received his Ph. D. degree from l’Université Louis Pasteur in Strasbourg, France, in 1995. After working in at the Max-Plank Institut fuer Kohlenforschung (1996-1997) and l’Università di Messina (1997-1998) as a post-doctoral Fellow, he started his academic career at the University of Waterloo in 1998 (Assistant Professor). In 2002 he moved to the Université de Montréal (as Associate Professor) where he is currently a Full Professor. He was recently awarded an Accelerator Grant and is currently the leader of a Strategic Project, both funded by the Natural Sciences and Engineering Research Council of Canada (NSERC). His current research interests include metal-assembled complexes, inorganic photochemistry, and photocatalytic hydrogen production.
Professor Sally Brooker (MNZM, FRSNZ, FNZIC, FRSC) studied at the University of Canterbury, New Zealand [BSc(Hons) first class; PhD with Professor Vickie McKee]. After postdoctoral research at Georg-August-Universität Göttingen, Germany, with Professor George M. Sheldrick, she took up a Lectureship at the University of Otago where she is now a full Professor and Sesquicentennial Distinguished Chair. She has been the recipient of numerous awards, most recently including a 2017 Queens Birthday Honour for services to science (MNZM), the 2017 Hector Medal (RSNZ) and 2017 Burrows Award (RACI). Her research interests concern the design, synthesis and full characterisation of, primarily paramagnetic, di- and poly-metallic complexes of transition metal and lanthanide ions with polydentate acyclic and macrocyclic ligands, as these have interesting redox, magnetic, catalytic and photophysical properties (otago.ac.nz/brooker).