The 2008 Nobel prize was awarded for the discovery and development of the green fluorescent protein (GFP). Since these initial discoveries almost 200 GFP-like Fluorescent proteins (FPs) have been described. FPs have become indispensable in biomedical and basic research as a genetically encoded fluorescent label due to the unique ability of the protein family to synthesize light-emitting chromophores autocatalytically from their own three amino acid residues situated near the center of the FP globule.
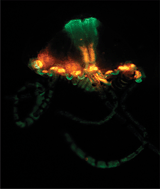
There has never been any wild-type GFP-related proteins found which contain multiple GFP-like domains within a single polypeptide chain. This paper describes two such proteins and explores possible relationships between individual domains within the four-domain orange-red protein from the anthoathecate jellyfish.
The results reveal a previously unrecognized direction in which natural FPs have diversified, suggesting new avenues to look for FPs with novel and potentially useful features.
Interested in knowing more? Read the full article for free until 15th February
Multi-domain GFP-like proteins from two species of marine hydrozoans
Marguerite E. Hunt, Chintan K. Modi, Galina V. Aglyamova, D. V. S. Ravikant, Eli Meyer and Mikhail V. Matz
Photochem. Photobiol. Sci., 2012, DOI: 10.1039/C1PP05238A