 In this pioneering study a team from Brazil and China have used site-directed mutagenesis of protoluciferase to better understand the structural evolution of luciferase activity.
In this pioneering study a team from Brazil and China have used site-directed mutagenesis of protoluciferase to better understand the structural evolution of luciferase activity.
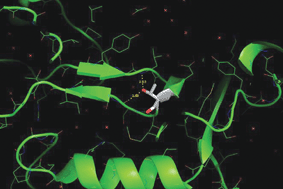
A major mystery in bioluminescence is how it arose during evolution. It is known that beetle luciferases evolved from AMP-CoA-ligases, also known as protoluciferases, which catalyse the activation of carboxylic acids. In this study a luciferase-like AMP-CoA-ligase from Zophobas morio mealworm was taken as a protoluciferase model. Site directed mutagenesis was used to replace residues in the carboxylic binding site with the respective ones conserved in beetle luciferases.
The team found that one substitution (I327T) improved the luminescence activity indicating the importance of the β-hairpin motif which it is contained in for bioluminescence activity in beetle luciferases. It also indicates a possible route for the evolution this activity.
Interested in knowing more? Read the full article for free until May 20th!
Structural evolution of luciferase activity in Zophobas mealworm AMP/CoA-ligase (protoluciferase) through site-directed mutagenesis of the luciferin binding site
R. A. Prado, J. A. Barbosa, Y. Ohmiya and V. R. Viviani
Photochem. Photobiol. Sci., 2011, Advance Article
DOI: 10.1039/C0PP00392A