Scientists in China have made a synthetic bone material using a new technique for creating polymer nanocomposites incorporated with inorganic nanoparticles.
Yuandong Dou, Kaili Lin and Jiang Chang, Nanoscale, 2011, DOI: 10.1039/C1NR10028A
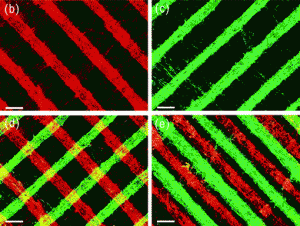
Jiang Chang and colleagues created this material after coming up with a new approach to making the nanocomposites, allowing them to control both the spatial distribution and orientational organisation of the nanocomponents, a known limitation of current methods of fabrication.
Their method involves using electrospinning and hot pressing techniques. They firstly homogeneously dispersed the nanoparticles within a polymer matrix solution, which was then electrospun into a patterned “nanofibrous mat” using a specifically designed “collector”. This mat was then placed between two sheets of non-woven polymer nanofibre and hot pressed to create the nanocomposite.
Because bone tissue is, generally speaking, structurally similar to these composites (they involve mineral particles preferentially oriented in a collagen matrix), the researchers tested their new fabrication method by creating an synthetic bone material by incorporating calcium silicate hydrate nanowires into a polyvinyl butyral matrix. Their artificial material showed remarkable mechanical properties, particularly when compared with the pure polymer (for instance, the bending strength of the researchers’ material reached 188 MPa, as compared to the 86 MPa of the polymer), which also matched those of real cortical bone tissue.
You can find out more about this work by reading the article here.










 HOT Nanoscale paper
HOT Nanoscale paper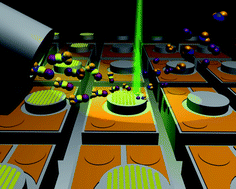

 ‘
‘