Researchers at Lanzhou University, China, have designed a nanocomposite to effectively remove cadmium ions from human blood.
Previous materials designed for this purpose have either had good selectivity, high saturation magnetisation or good water dispersibility, but the new material has all three properties. And, the composite is highly supermagnetic, making subsequent removal of the nanoparticles easier.
Removing cadmium (which is produced during industrial processes) from the blood is important because they bind to proteins in the body, affecting their functions.
The nanocomposite consists of four components; The first is magnetic iron oxide nanoparticles, chosen for their low toxicity. They are coated with polyethylenimine to increase the amino groups on the particles’ surface to bind Cd2+, but also to lower nanoparticle uptake by red blood cells, maximising the circulation time of the composites in the blood. Polyethylene glycol is grafted onto this as an anchor for negatively charged 2,2’-phenylazanediyl, which counteracts the hydrophobic and electrostatic interactions between the nanoparticles and plasma proteins or white blood cells.
Read the Nanoscale article now:
2, 2′-(phenylazanediyl) diacetic acid modified Fe3O4@PEI for selective removal of cadmium ions from blood
Jun Jin, Fang Yang, Fengwei Zhang, Wuquan Hu, Shao-bo Sun and Jiantai Ma
Nanoscale, 2012
DOI: 10.1039/c2nr11481j






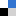




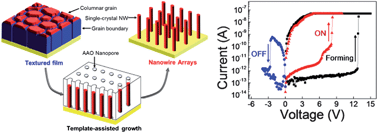
 Read this highly topical feature review article today:
Read this highly topical feature review article today:
