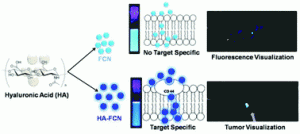
An in-situ synthesis of biocompatible fluorescent carbon nanoparticles (FCNs) is reported for targeted bioimaging. The nanoparticles were formed via dehydration of hyalurinic acid (HA), and through careful alteration of the carbonisation times, the total content of HA and fluorescence in the carbon nanoparticles could be controlled. Sharker et al. then compared two colloidally stable FCN samples; one partially carbonised sample that still contained some HA (HA-FCN), against a “non-specific” fully carbonised sample containing no HA (FCN).
Before in vivo testing, both sets of particles were tested on different cell lines at dosages up to 1.0 mg/ml and were found to not affect cell viability. Interestingly, HA-FCNs showed more uptake than the non-specific FCNs, and were internalised more various cell lines; including cancer cells. This is speculated to be due to the over expression of the CD-44 receptor which can facilitate uptake of particles containing targeting molecules such as HA-FCNs. In vivo bio-distribution studies showed more accumulation of HA-FCNs in tumours pre-implanted into mice compared to FCNs, when particles were injected into the tail vein. This is expected to be of enormous potential in not only bioimaging, but also drug delivery and diagnostics.
In situ synthesis of luminescent carbon nanoparticles toward target bioimaging
Shazid Md. Sharker, Sung Min Kim, Jung Eun Lee, Ji Hoon Jeong, Insik In, Kang Dea Lee, Haeshin Lee and Sung Young Park
Nanoscale, 2015, 7, 5468-5475. DOI: 10.1039/C4NR07422J
Dr Mike Barrow is a guest web writer for the Nanoscale blog. He currently works as a Postdoctoral Researcher at the University of Liverpool.