New Nanoscale communication
Carl A. Otter, Philipus J. Patty, Martin A. K. Williams, Mark R. Waterland and Shane G. Telfer
Nanoscale, DOI: 10.1039/c0nr00801j
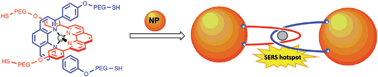
A specially designed metallosupramolecular linker has been used to ‘interlock’ gold and silver nanoparticles into aggregate assemblies. Waterland, Tefler and coworkers at Massey University in New Zealand used a PEGthiol-functionalised bis(phenanthroline)copper(I) complex which acted to ‘catenate’ the nanoparticles into mechanically interlocked structures, which formed a stable yet chemically modifiable linking mechanism with well defined particle separation.
The mechanism behind this particle interlocking is simple and extremely effective (see figure). The copper(I) centre in the complex arranges two phenanthroline ligands in an orthogonal arrangement., while two phenyl substituents direct the polyethylene glycol (PEG) chains away from each side. The PEG chains, which bestow water solubility on the complex, are terminated with thiol groups which have a strong affinity for the nanoparticle surfaces. Once these thiol groups are attached to the nanoparticles, the system locks two particles together into a physically entwined aggregate. The group employed DLS, SERS and TEM in their analyses, three complementary experimental techniques which allowed them to develop a comprehensive picture of their interlocking nanoparticle systems.
The controlled assembly of nanoparticles into complex structures is extremely important in the quest to design and synthesise complex, efficient and multifunctional nanostructures. Strategies such as those employed in this work, which combines the exciting fields of supramolecular chemistry and nanoparticle design, will be extremely important in the future development of novel nanotechnologies.
To read this communication, click here.