To address the need for large-scale electrochemical energy storage (EES), much research attention has moved beyond Li-ion batteries due to safety and security-of-supply issues. Sodium, an alkali-metal which is much more abundant and well-distributed globally, is of keen interest as its mining process is cleaner and freer from ethical concern. Moreover, to avoid the high flammability of organic electrolytes, some researchers are looking towards aqueous sodium-ion batteries as a potential contender for future EES systems. This has the added benefit of increasing the ionic conductivity by as much as two orders of magnitude versus organic equivalents, potentially enabling higher rate capability. However, moving from traditional carbonate-based electrolytes to water means a narrowing of the electrochemical stability window and the need for electrodes to facilitate the intercalation and de-intercalation of hydrated cations. Given the smaller accessible voltage and the larger charge carrier, aqueous sodium-ion batteries are still plagued by low specific energy and limited lifespans.
Therefore, the development of new electrode materials to maximise specific capacity is an important research direction. For the anode material, much attention has been paid to the development of polyanionic materials, such as sodium superionic conductors (NASICON), but also carbon-based materials such as polypyrrole and polyimide systems. In recent work by Maria K. Ramos et al., however, the synthesis of a graphene-based composite thin-film was presented, incorporating two compounds that had been shown to have high capacities but suffered from low conductivity and significant volume changes.
Specifically, the researchers highlighted the difficulty of producing ternary thin-films by traditional fabrication routes (e.g., spin-coating, vapour deposition etc.), spurring the development of a liquid-liquid interfacial route (LLIR) for the self-assembly of materials at the interface of immiscible liquids to give a continuous network that can be deposited on any solid substrate. MoS2, known to facilitate the intercalation and de-intercalation of hydrated sodium ions, and non-toxic copper oxide nanoparticles with high theoretical specific capacity, were combined with graphene in this way to produce ternary films that were electrochemically characterised.
Interestingly, the researchers detailed three different thin-film preparation approaches using their LLIR method (Figure 1). The in-situ approach, whereby graphene oxide and Cu2+ were simultaneously reduced in a dispersion of MoS2, yielded a thin-film anode material that demonstrated a very high specific capacity of 1377 mA h g-1 (c.f. specific capacity of typical lithium-ion batteries is < 200 mA h g-1).
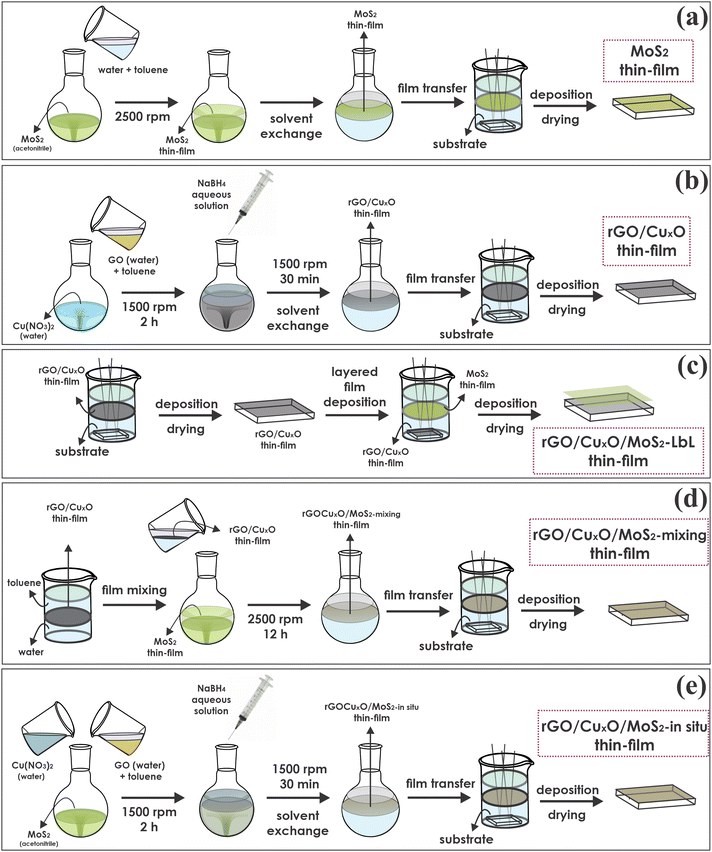
Figure 1: Schematic representation of the general steps for thin-film preparation of: (a) MoS2; (b) rGO/CuxO or rGO; (c) rGO/MoS2 and rGO/CuxO/MoS2 layer-by-layer; (d) rGO/MoS2 and rGO/CuxO/MoS2 mixing; and (e) rGO/MoS2 and rGO/CuxO/MoS2 by an in-situ method. Reproduced from DOI: 10.1039/d3mh00982c with permission from the Royal Society of Chemistry.
In summary, the successful implementation of this in-situ liquid-liquid interfacial method for thin-film preparation provides encouragement for its use to produce other composite electrode materials, and a greater understanding of its scalability. The demonstration of such a high-capacity aqueous sodium-ion battery electrode should encourage greater exploration of this more sustainable, beyond-lithium EES technology.
To find out more, please read:
Nanoarchitected graphene/copper oxide nanoparticles/MoS2 ternary thin films as highly efficient electrodes for aqueous sodium-ion batteriesMaria K. Ramos, Gustavo Martins, Luiz H. Marcolino-Junior, Márcio F. Bergamini, Marcela M. Oliveira and Aldo J. G. Zarbin
Mater. Horiz., 2023, 10, 5521-5537, DOI: 10.1039/d3mh00982c
About the blogger

Dr Josh J Bailey is an Illuminate Fellow at Queen’s University Belfast, focused on the implementation and optimisation of ionic liquids used in polymer electrolyte fuel cells and is a Materials Horizons Community Board member. He received his doctoral degree from University College London, UK, as part of the Centre for Doctoral Training in Advanced Characterisation of Materials, investigating electrode degradation in solid oxide fuel cells. His research interests span fuel cells, lithium-ion batteries, solid-state batteries, and flow batteries, both in terms of the design of novel electrodes, electrolytes, and membrane materials, as well as the study of materials degradation, with a view to improving performance and durability. |