In the realm of large-scale energy storage, the quest for low-cost, high-energy battery technologies has spurred the emergence of various alternatives. Lithium batteries utilizing Co-free conversion-type cathodes, alongside multivalent cation, and halogen anion batteries, stand out among the contenders. Conversion-type cathodes like iron fluorides in lithium batteries offer cost efficiency, higher capacity, and higher energy density compared to cathodes containing expensive transition metals. However, the use of lithium metal anodes presents safety and cost challenges, inhibiting effective real-world battery performance. Similarly, while multivalent cation batteries, such as those based on Mg2+, boast abundant reserves, their strong coulombic interactions with host materials create challenges with charge carrier migration. To address these issues and develop batteries with favourable reaction kinetics and reversibility, the burgeoning field of halogen anion batteries, particularly fluoride ion batteries (FIBs), holds promise.
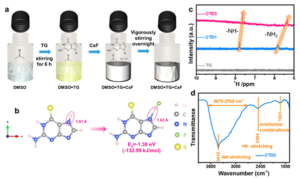
Fig. 1 Preparation and characterization of electrolytes. (a) Preparation process of the CTD3 electrolyte. (b) Illustration of adsorption of the TG molecule to F and its adsorption energy. (c) 1 H NMR spectra of TG, CTD1 and CTD3. (d) FT-IR spectrum of CTD3. Reproduced from DOI: 10.1039/D3MH01039B with permission from the Royal Society of Chemistry.
FIBs, leveraging the unique properties of fluorine as the lightest and most electronegative element among halogens, offer the highest theoretical energy density. Despite this potential, realizing practical applications has been hindered by the lack of suitable electrolytes with high ionic conductivity at room temperature. The insolubility of fluoride salts in aprotic solvents has been a primary challenge. While boron-based anion acceptors (AAs) aid in dissociating fluoride salts, their strong Lewis acidity impedes fluoride transport, leading to unsatisfactory electrolyte conductivity. Addressing this limitation, a novel AA with mild Lewis acidity has been developed, facilitating fluoride salt dissociation while avoiding strong AA-F bonding. This breakthrough enables prepared electrolytes to achieve high ionic conductivity, reaching up to 2.4 mS cm-1 at room temperature, enabling successful FIB operation with a reversible capacity of 126 mA h g-1 after 40 cycles.
Moreover, understanding the regulation effect of salt concentration on the cathode interface has unveiled insights into improving FIB performance, emphasizing the critical role of rational electrode-electrolyte interface design in future FIB development. FIBs hold significant promise owing to their potential for high energy density and favourable compatibility with high-voltage electrode materials. Notably, fluorine’s abundance—two orders of magnitude higher in global production than lithium—further accentuates their appeal. Conversion-type FIBs, with a theoretical energy density of 5000 W h L-1, exhibit substantial energy density even at leaner stack levels, offering a cost as low as $20 per kW h-1 according to techno-economic analysis. Despite these merits, the experimental realization of the remarkable energy density of FIB is hindered by the lack of well-tailored electrolytes with suitable ionic transport abilities and electrochemical stability.
Liquid electrolytes for FIBs have garnered interest due to their high room-temperature ionic conductivity and better wettability compared to solid-state electrolytes. However, challenges persist, mainly the insolubility of fluoride salts in regular organic aprotic solvents due to strong electrostatic interactions. To address this, efforts have been directed toward designing softer Lewis acidity AAs that facilitate fluoride salt dissolution without excessive solvation, crucial for practical liquid electrolytes. Innovations in this work have introduced a novel sulfone electrolyte based on a new molecular-type H-donor AA (6-thioguanine, TG) with moderate Lewis acidity. Demonstrated through various analyses, this electrolyte achieves impressive ionic conductivity at room temperature, enabling the reversible cycling of FIBs. The superior reversibility is attributed to the electrolyte’s high ionic conductivity, improved desolvation capability of fluoride ions, and a well-designed interface layer.
In summary, the pioneering advancements in electrolyte design for fluoride ion batteries set the stage for increasingly viable and effective energy storage solutions, offering improved reversibility and reliable performance at ambient temperatures.
To find out more, please read:
Room-temperature reversible F-ion batteries based on sulfone electrolytes with a mild anion acceptor additive
Yifan Yu, Meng Lei and Chilin Li
Mater. Horiz., 2023, Advance Article, DOI: 10.1039/D3MH01039B
About the blogger

Edison Huixiang Ang serves as an Assistant Professor at the National Institute of Education/Nanyang Technological University, Singapore, and a member of the Materials Horizons Community Board. Dr. Edison specializes in nanotechnology, particularly exploring 2D nanomaterials for applications in energy storage, membrane technology, catalysis, and sensors. Stay updated on his work by following him on X (formerly Twitter) @edisonangsg. |