2014 has arrived and with it a new batches of Hot Articles, one of which from January deserves special attention. Professor Sunny Chan‘s group at Academia Sinica, Taiwan have achieved the distinction of being the first group to devise a molecular catalyst for the selective oxidation of methane to methanol. This reaction faces a formidable challenge in the form of inertness of the methane C–H bond which makes O-atom insertion into the molecule almost impossible in ambient conditions. Even if this problem is solved, the product, methanol, is highly susceptible to over-oxidation leading to formation of other undesired products. For of these reasons, most of the researchers have failed to scale this gargantuan mountain of difficulties.
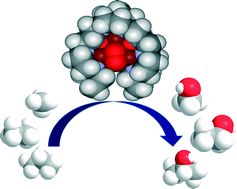
Time and again when scientists have found it difficult to get answers to tough and challenging problems they have turned to nature for inspiration. In this case, the solution lay in a particular class of enzymes called methane monoxygenases (MMO) found in the methanotrophic bacteria. These MMOs have metallic clusters at their centres, which catalyse this difficult reaction with ease. In order to emulate these catalytic centres, the researchers developed some biomimetic models containing tricopper clusters, one of which, [CuICuICuI(7-N-Etppz)][ClO4], successfully mediated the selective oxidation of methane without any over-oxidation. This tricopper complex, when activated by dioxygen (O2), harnesses a “singlet oxene”, the strongest oxidant that could be used for a facile O-atom insertion across the C-H bond.
Biomimetic Tricopper complex as a catalyst for selective oxidation of methane to methanol
The catalyst also gave selectivity in the cases of ethane and propane, but not with higher alkanes. The reason being is the design of the tricopper catalyst, which has a small hydrophobic binding pocket at the base and forms a transient complex with the alkane and carries out the oxene transfer to oxidize the substrate. This pocket is not big enough to accommodate the product methanol (as well as the other small alcohols), so it releases the product as soon as it is formed. This removes over-oxidation from the equation, giving profound selectivity in cases of smaller alkanes. The authors have further studied the catalytic cycles and analysed the factors affecting the catalytic turnovers and efficiency.
This work presents a move towards a more efficient flow system which, in the future, would help in increasing the yields of the products. One issue with the current system is the solubility of the catalyst in solvents which can dissolve CH4 gas which may be put to rest by some modification in the design of the catalyst, leaving brighter prospects for the future.
To find out more about this nature-inspired discovery, read the full article now for more details.
Developing an efficient catalyst for controlled oxidation of small alkanes under ambient conditions
Penumaka Nagababu, Steve S.-F. Yu, Suman Maji, Ravirala Ramu and Sunney I. Chan
Catal. Sci. Technol., 2014, DOI: 10.1039/C3CY00884C
 Shreesha Bhat is a M.S.(Pharm.) in Medicinal Chemistry from National Institute of Pharmaceutical Education and Research, India. He has recently joined the research group of Dr. Pallavi Sharma as a PhD student at the University of Lincoln, UK. His project involves the design and synthesis of Helicase-primase inhibitors for Herpes Simplex virus and development of useful synthetic methodologies.
Shreesha Bhat is a M.S.(Pharm.) in Medicinal Chemistry from National Institute of Pharmaceutical Education and Research, India. He has recently joined the research group of Dr. Pallavi Sharma as a PhD student at the University of Lincoln, UK. His project involves the design and synthesis of Helicase-primase inhibitors for Herpes Simplex virus and development of useful synthetic methodologies.