It gives an absolute delight to the grey matter to imagine power generated from simple tap water. Power which could be supplied to our home and cars and thus put an end to the incessant use of fossil fuels. Yes friends, after the Bronze and Iron age, now it’s time for the Hydrogen age. The term “Hydrogen Economy” is gaining momentum and has the potential to do for the energy revolution what the computer and the Internet have done for the information revolution.
Among the various methods available for hydrogen production, water-splitting is one of the most promising approaches. Earlier, water oxidation catalysis have been performed efficiently with expensive, toxic and earth-scarce transition metals, but 3d metal-based catalysts are much less established. Fillol and Costas in their Nature Chemistry paper explored the use of environmentally benign and easily available iron coordination complexes for water oxidation with evident success. Their observation suggested that the iron complexes, when combined with Ce(IV) gets decomposed to iron oxides, which are, in fact, the main active catalysts for the water oxidation.
Substantial work by Tai-Chu Lau and group indicated that the actual catalysts for water oxidation are different at low and high pH values. It has been confirmed through various studies that at high pH, Fe2O3 is indeed the active metal catalyst, but researchers are still mystified as to what would be the intermediate at low pH. It has been speculated that the water oxidation at low pH goes through a molecular oxo-Fe active intermediate, as no evidence for Fe oxide formation has been obtained till date. The reason for this was given by Fillol and Costas, who proved that Fe (III) does not oxidize water in acidic conditions nor does it convert to Fe oxide. Another possibility that has been looming in the minds of the scientists is that FeO42- ions being strong oxidants can oxidize water in acidic conditions. But, scientists have shown that Ce(IV) is not capable of oxidizing Fe(III) to FeO42–. So, It has been a matter of debate as to which is the real catalyst for low pH water oxidation: Is it FeO42- indeed? Or an oxo-Fe intermediate?
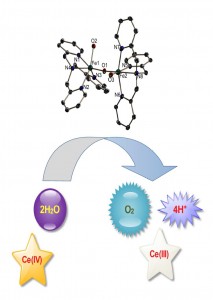
Water oxidation by iron complexes in presence of Ce(IV)
In this communication, a group of Iranian scientists have tried to answer this question and included it in their quest for a more effective iron-based water oxidation catalyst. Their work has substantial basis in the work done by Fillol and Costas whose one observation was the inability of Fe(III) oxohydroxo 2µ-(O,OH) diferric dimer to catalyze water oxidation. So, a question was posed by Mohammad Mahdi Najafpour and group if an Fe(III) complex with only one bridge (oxo {O} or hydroxo {OH}) can be a water oxidizing catalyst? And if FeO42- has any role in the whole process?
To answer the above two important questions, they synthesized an Fe(III) oxo diferric dimer with tris(2-pyridylmethyl)amine (tpa) ligand with only one µ-O bridge, and tested the dimer for water oxidation in presence of Ce(IV), and found it to be 6 times more active (measured in terms of TOF) than the monomer reported by Fillol, Costas and workers. This provided a new insight into the mechanism, suggesting that a monomer might be just a precursor to the active catalyst which might be a di- or a multinuclear iron compound. The experiments prove that even if Fe ions convert to FeO42- in the presence Ce(IV), FeO42- cannot oxidize water catalytically, thus putting an end to the long lasting debate of FeO42- being an intermediate in these reactions.
Read more at:
A dinuclear iron complex with a single oxo bridge as an efficient water-oxidizing catalyst in the presence of cerium (IV) ammonium nitrate: New findings and current controversies
Mohammad Mahdi Najafpour, Atefeh Nemati Moghaddam, Davood Jafarian Sedigh and Malgorzata Holynska
Catal. Sci. Technol.,2013, Accepted Manuscript
DOI: 10.1039/C3CY00644A
 Shreesha Bhat is a M.S.(Pharm.) in Medicinal Chemistry from National Institute of Pharmaceutical Education and Research, India. He has recently joined the research group of Dr. Pallavi Sharma as a PhD student at the University of Lincoln, UK. His area of interests include chemical synthesis of biologically important molecules and developing newer methods for organic synthesis using novel catalysts.
Shreesha Bhat is a M.S.(Pharm.) in Medicinal Chemistry from National Institute of Pharmaceutical Education and Research, India. He has recently joined the research group of Dr. Pallavi Sharma as a PhD student at the University of Lincoln, UK. His area of interests include chemical synthesis of biologically important molecules and developing newer methods for organic synthesis using novel catalysts.










