This month sees the following articles in Catalysis Science & Technology that are in the top ten most accessed:-
Graphene-based materials for catalysis
Bruno F. Machado and Philippe Serp
Catal. Sci. Technol., 2012,2, 54-75 DOI: 10.1039/C1CY00361E
A review of controllable synthesis and enhancement of performances of bismuth tungstate visible-light-driven photocatalysts
Liwu Zhang and Yongfa Zhu
Catal. Sci. Technol., 2012,2, 694-706 DOI: 10.1039/C2CY00411A
Conversion of lignocellulose into renewable chemicals by heterogeneous catalysis
Hirokazu Kobayashi , Hidetoshi Ohta and Atsushi Fukuoka
Catal. Sci. Technol., 2012,2, 869-883 DOI: 10.1039/C2CY00500J
Role of mixed metal oxides in catalysis science—versatile applications in organic synthesis
Manoj B. Gawande , Rajesh K. Pandey and Radha V. Jayaram
Catal. Sci. Technol., 2012,2, 1113-1125 DOI: 10.1039/C2CY00490A
Design of hierarchical zeolite catalysts by desilication
Danny Verboekend and Javier Pérez-Ramírez
Catal. Sci. Technol., 2011,1, 879-890 DOI: 10.1039/C1CY00150G
Fischer–Tropsch reaction–diffusion in a cobalt catalyst particle: aspects of activity and selectivity for a variable chain growth probability
David Vervloet , Freek Kapteijn , John Nijenhuis and J. Ruud van Ommen
Catal. Sci. Technol., 2012,2, 1221-1233 DOI: 10.1039/C2CY20060K
Rational design of heterogeneous catalysts for biodiesel synthesis
Karen Wilson and Adam F. Lee
Catal. Sci. Technol., 2012,2, 884-897 DOI: 10.1039/C2CY20038D
Asymmetric catalytic carbon–carbon coupling reactions via C–H bond activation
Lei Yang and Hanmin Huang
Catal. Sci. Technol., 2012,2, 1099-1112 DOI: 10.1039/C2CY20111A
Challenge and progress: palladium-catalyzed sp3 C–H activation
Hu Li , Bi-Jie Li and Zhang-Jie Shi
Catal. Sci. Technol., 2011,1, 191-206 DOI: 10.1039/C0CY00076K
Structure and catalytic properties of hexagonal molybdenum disulfide nanoplates
Carlos Fernando Castro-Guerrero , Francis Leonard Deepak , Arturo Ponce , Juan Cruz-Reyes , Mario Del Valle-Granados , Sergio Fuentes-Moyado , D. H. Galván and Miguel José-Yacamán
Catal. Sci. Technol., 2011,1, 1024-1031 DOI: 10.1039/C1CY00055A
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Catalysis Science & Technology? Then why not submit to us today or alternatively email us your suggestions.
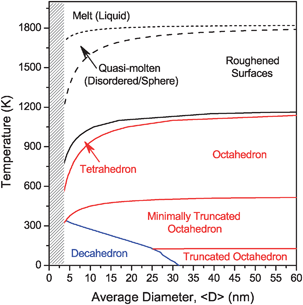
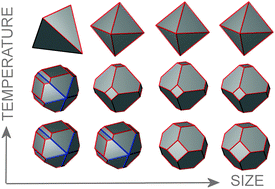 There is so much research currently being dedicated to nanoparticle catalysts but how can we plan for how they will behave under different temperature conditions?
There is so much research currently being dedicated to nanoparticle catalysts but how can we plan for how they will behave under different temperature conditions?