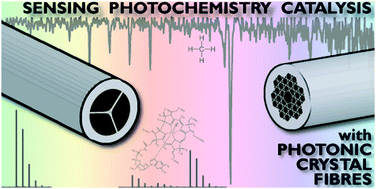
Photonic crystal fibres for chemical sensing and photochemistry
Ana M. Cubillas, Sarah Unterkofler, Tijmen G. Euser, Bastian J. M. Etzold, Anita C. Jones, Peter J. Sadler, Peter Wasserscheid and Philip St.J. Russell
Chem. Soc. Rev., 2013, Advance Article
DOI: 10.1039/C3CS60128E, Review Article
Part of our upcoming themed issue: Chemical and biological detection
Free to access until 20th October 2013
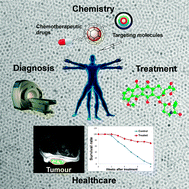
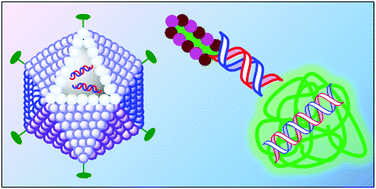
Magnetic nanoparticles as contrast agents in the diagnosis and treatment of cancer
Juan Gallo, Nicholas J. Long and Eric O. Aboagye
Chem. Soc. Rev., 2013,42, 7816-7833
DOI: 10.1039/C3CS60149H, Review Article
Free to access until 20th October 2013
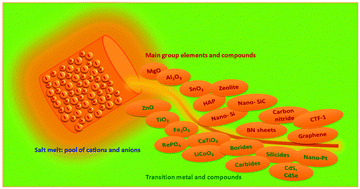
Salt melt synthesis of ceramics, semiconductors and carbon nanostructures
Xiaofeng Liu, Nina Fechler and Markus Antonietti
Chem. Soc. Rev., 2013, Advance Article
DOI: 10.1039/C3CS60159E, Review Article
Free to access until 20th October 2013
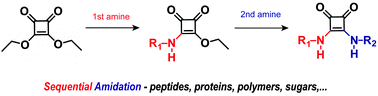
Be squared: expanding the horizon of squaric acid-mediated conjugations
Frederik R. Wurm and Harm-Anton Klok
Chem. Soc. Rev., 2013, Advance Article
DOI: 10.1039/C3CS60153F, Review Article
Free to access until 20th October 2013
Chemistry and formulations for siRNA therapeutics
Andrzej Gallas, Cameron Alexander, Martyn C. Davies, Sanyogitta Puri and Stephanie Allen
Chem. Soc. Rev., 2013, Advance Article
DOI: 10.1039/C3CS35520A, Tutorial Review
Free to access until 20th October 2013
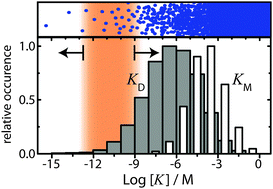
Breaking the concentration limit of optical single-molecule detection
Phil Holzmeister, Guillermo P. Acuna, Dina Grohmann and Philip Tinnefeld
Chem. Soc. Rev., 2014, Advance Article
DOI: 10.1039/C3CS60207A, Tutorial Review
Part of our upcoming themed issue: Single-molecule optical spectroscopy
Free to access until 20th October 2013
THAT’S NOT ALL! Click here for more free HOT Chem Soc Rev articles for June-August!