Friedrich Wöhler’s early syntheses of oxalic acid and urea heralded the age of synthetic organic chemistry. These reactions demonstrated the potential for man to generate compounds that had previously only been obtained from the extraction of biological substances. Remarkably, despite huge advances in chemical synthesis, almost all natural products synthesised to date have relied upon similar apparatus and techniques to those used by Wöhler in the 1820s. Steve Ley and his group are among the pioneers of the use of flow chemistry in synthesis, and have demonstrated the use of machines in place of the antiquated round-bottomed flasks still used in chemistry labs the world over.
In flow chemistry (at its most basic), a reaction is performed in a continuous flowing stream where substrates and reagents are combined inside inert tubing and pumped around a coil of tubing before being quenched or treated with the chemical required for the next stage of the transformation.
Ley and coworkers have recently published a review that presents some highlights from the use of flow chemistry in natural product synthesis. One of the notable examples featured in this review is the continuous flow, semi-synthesis of artemisinin by Seeberger and Lévesque. Artemisinin is a sesquiterpene that represents the frontline treatment for plasmodium falciparum malaria when used in combination with other therapeutics. The supply of artemisinin from natural sources is problematic as is the scalability of existing synthetic approaches.
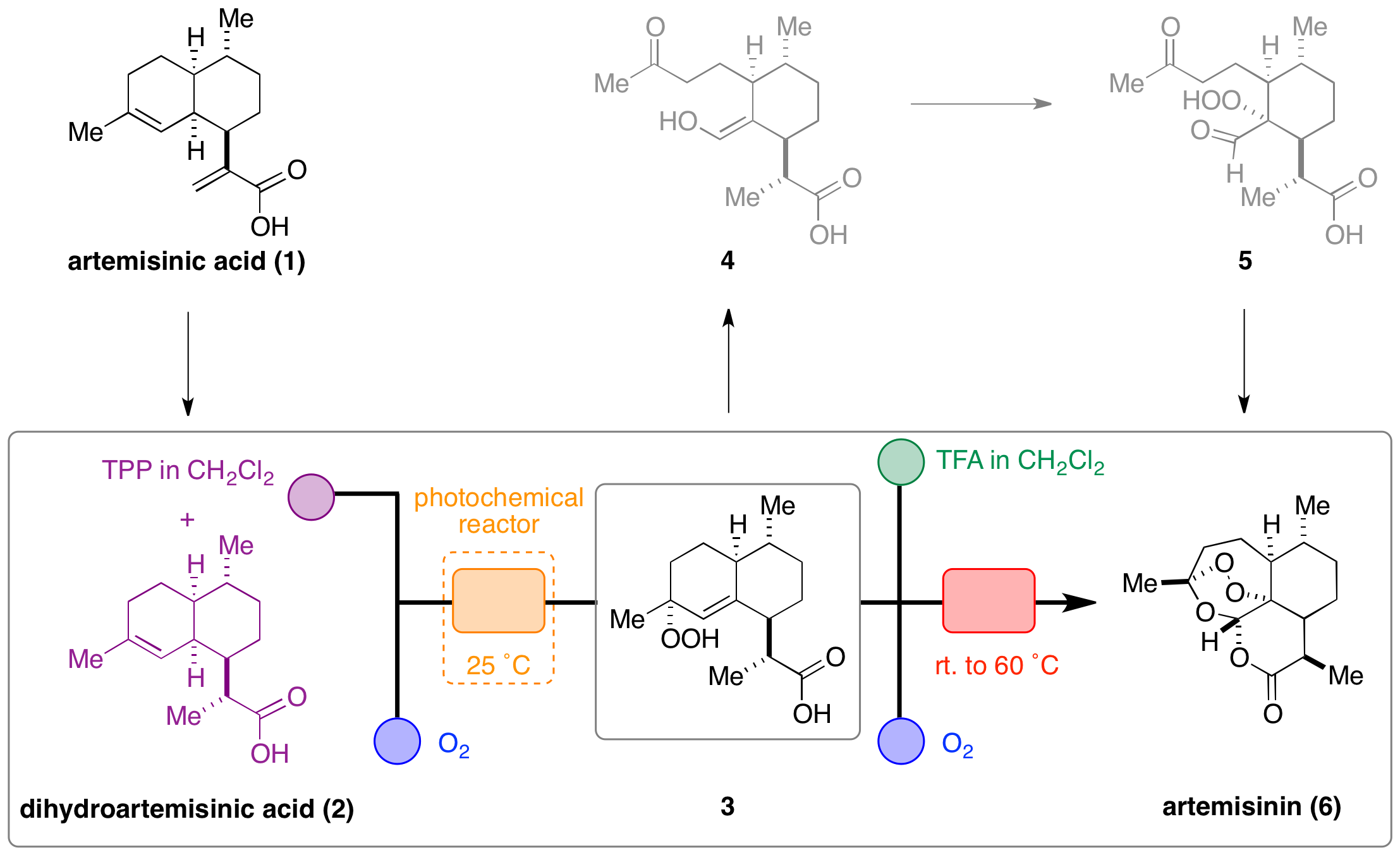
Dihydroartemisinic acid 2, (derived from artemesinic acid 1) represents the starting point for this flow synthesis and first undergoes photooxidation to yield hydroperoxide 3. Subsequent treatment of 3 with strong acid, followed by oxidation provided hydroperoxide 5, which underwent a spontaneous cycloaddition sequence, leading to the generation of artemisinin 6.
The use of a continuous flow reactor particularly enhanced the challenging photochemical transformations associated with the synthesis. Issues such as low mass transfer of oxygen gas into solution and low penetration of light were resolved by coiling the reaction tubing around a lamp to enabled effective generation of the singlet-oxygen required for the reaction. Additionally, improved mixing and temperature control could also be achieved. Crucially, this synthesis provides a low cost method to meet the escalating demand for artemisinin at affordable prices for patients in the developing world.
The elegant syntheses described in this review span a range of natural product classes and highlight the advantages that mechanisation of chemical processes can offer. As chemists seek to address medicinal and environmental challenges, perhaps greater emphasis should be placed on rational design rather than labour-intensive and repetitive tasks. The effective implementation of flow systems and technology could revolutionise the chemical sciences, and this review provides some exciting food for thought.
For more, read this HOT Chem Soc Rev article in full:
Flow chemistry syntheses of natural products
Julio C. Pastre, Duncan L. Browne and Steven V. Ley
Chem. Soc. Rev., 2013, Advance Article
DOI: 10.1039/C3CS60246J
Alice Williamson is a guest web-writer for Chem Soc Rev. She is currently a postdoc for the OSDDMalaria Project in Dr. Matthew H Todd’s group at the University of Sydney.