 This review article by Chen et al., based at Peking University in Beijing, encompasses a wide range of recent work on bioorthogonal protein chemistry mediated by transition metals in living cells.
This review article by Chen et al., based at Peking University in Beijing, encompasses a wide range of recent work on bioorthogonal protein chemistry mediated by transition metals in living cells.The definition of bioorthogonal chemistry is a reaction which can take place inside a living system without disrupting the biological processes surrounding it. The reactants must be inert to any molecules or biomolecules in the surrounding environment, selective in their reaction with each other and non-toxic. The aim of bioorthogonal reactions is to label or influence a key biomolecule so that either it, or its effects, can be tracked. Successful bioorthogonal reactions will allow for the study of biomolecules such as proteins within living systems, in real time. This review focuses on recent work by groups who have significantly contributed to protein chemistry and have widened the scope of bioorthogonal reactions.
Unsurprisingly, most reaction conditions do not meet the strict criteria of bioorthogonal reactions. The use of transition metals is discussed with a focus on the well-known Cu(I)-catalysed azide-alkyne cycloaddition (CuAAC) which is described as a ‘hallmark of bioorthogonal chemistry’ due to its high specificity, reaction rate and selectivity. The triazole product is stable and unreactive but this reaction is prevented from being bioorthogonal by the toxicity of the Cu(I) ions.
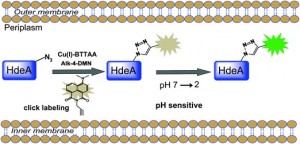
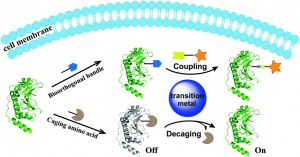
Chen and co-workers review research focused on overcoming the toxicity problems seen with Cu(I), including the strain-promoted azide-alkyne cycloaddition (SPAAC). This work developed by Bertozzi et al. is a ‘copper-free’ version of the CuAAC reaction. Chen et al. additionally discuss their own work, on ligand-assisted CuAAC reactions, using a BTTAA ligand (2-[4-({bis[(1-tert-butyl-1H-1,2,3-triazol-4-yl)methyl]amino}methyl)-1H-1,2,3-triazol-1-yl]acetic acid) to reduce toxicity. The authors also assess the use of other transition metals, such as palladium, as replacements for copper in bioorthogonal conjugation reactions, both on the cell surface and within an intracellular environment.
Ligand-assisted CuAAC labelling of proteins in the bacterial periplasm.
To download the full article for free* click the link below:
Maiyun Yang, Jie Li and Peng R. Chen
DOI: 10.1039/C4SC00646A
This article is part of the Chem Soc Rev Emerging Investigators themed issue, which showcases up-and-coming scientists who are internationally recognised for making outstanding contributions to their respective fields.
*Access is free through a registered RSC account – click here to register