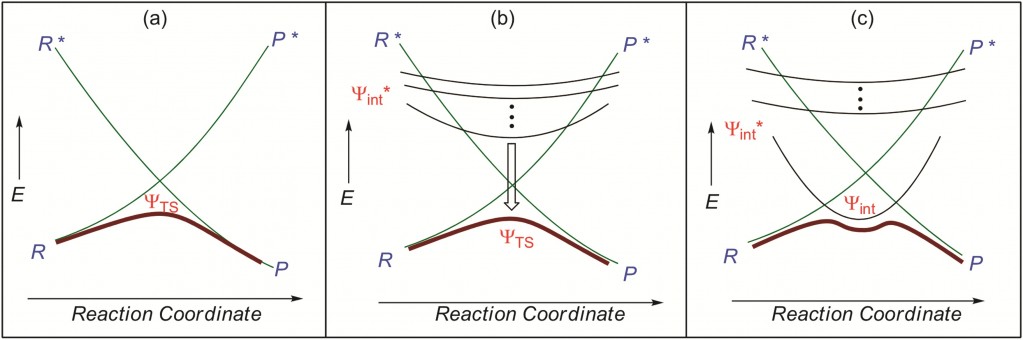
Chemical reactions often have reaction barriers that must be overcome in order for reactants to become products. Appreciating the origins of these barriers and more importantly quantifying their heights from raw data is of significant use to the Chemist. Therefore, the Chemist would like to have these features in the general model of reactivity which they use. A model that can predict barriers from raw data is the Valence Bond model, the focus of this quality Tutorial Review.
Sason Shaik from The Hebrew University of Jerusalem and colleagues share with the reader their insight from the development of the Valence Bond model. They focus on hydrogen atom transfer, the step most chemical oxidations begin with and which is therefore immensely important. They begin from the simplest hydrogen exchange reaction and work up to the more complex hydroxylation by Cyctochrome P450.
Valence Bond Models and the effect of different intermediates on the energy profile (bold line).
The authors take the reader through the preparation and use of valence bond diagrams and thus equip the reader with the tools required to understand mechanisms and predict chemical reactivity patterns. The authors have taken their role as tutors seriously and have provided the reader with supplementary data which they can use to work through problems and reconstruct results on their own. This focus on the reader, as a student, is very welcome and will ensure the interested reader appreciates the quality of the Valence Bond model as a useful interface between experiment and theory and between computations and understanding.
Read the Chem Soc Rev Tutorial Review in full now – for free*
A Tutorial for Understanding Chemical Reactivity Through The Valence Bond Approach
Dandamudi Usharani, Wenzhen Lai, Chunsen Li, Hui Chen, David Danovich and Sason Shaik
Chem. Soc. Rev., 2014, advance article
DOI: 10.1039/C4CS00043A
*Access is free through a registered RSC account – click here to register