Escalating global energy demand along with the limited supply of fossil fuels and mandates to minimise CO2 emissions has increased demand for alternative energy sources. Li-ion batteries have played a key role competing with Ni-MH batteries to supply power for small electronics since Sony launched the first generation Li-ion battery in the early 1990s. Recently, Li-ion batteries have predominantly provided the electrical power necessary to operate small portable electronic devices such as cellular phones, laptop computers, and camcorders. In addition, they have been used in both hybrid electric vehicles (HEVs) and back-up electricity storage units for renewable energy sources which require a large unit.
In 2008, sales of rechargeable Li-ion batteries reached 10 billion dollars and are currently growing at nearly 10 per cent per year. Furthermore sales are expected to grow dramatically if Li-ion batteries can be successfully implemented in HEVs or plug-in electric vehicles (PEVs).
A Li-ion battery is mainly composed of a carbonaceous anode (generally graphite), a carbonate-based organic electrolyte with a Li-containing salt (e.g. LiPF6), and a Li metal oxide cathode (generally LiCoO2). Li ions are inserted between graphite and LiCoO2 through the electrolyte during charge and discharge, respectively. Since the demand for safe Li-ion batteries exhibiting high power, large capacity, and high rate capability is ever increasing, research has been carried out worldwide to find new electrode materials to replace the currently used materials.
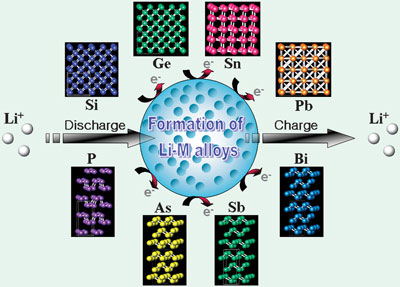
Li metal can electrochemically alloy and de-alloy with other metals at room temperature in an organic electrolyte electrochemical cell. Li-alloying reactions with metallic or semi-metallic elements and various compounds have been investigated during the past few decades. Although these alloying materials provide a larger specific capacity than graphite, except for a few transition metal oxides, they generally suffer from a large irreversible capacity at the first cycle and poor cycling behavior due to a large volume change during cycling.
If the microstructure of the electrode materials can be designed properly, the volume change during lithiation and delithiation would be compromised to some extent. In 1997, Fuji announced its Stalion battery, which employed an amorphous tin composite oxide (TCO) anode, but it was not commercialised because of its large irreversible capacity during the first cycle. Since then, Sony developed its Nexelion battery in 2005 using an anode material mainly composed of a Sn/Co/C composite with Ti metal synthesised by a high energy mechanical milling process. Not only composite materials, but also nanosized particles and nanostructured materials have also been suggested to alleviate the mechanical strain generated due to the volume change as the Li ions are inserted to and extracted from the host electrode materials.
A number of scientists have examined alloy-based anodes, in particular, focusing on the Group IV and Group V elements and their composites for Li-ion batteries. Research on Group IV elements has been performed using several material concepts based on nano-architecturing of materials, active/inactive composite, intermetallic compound, and the use of carbonaceous material as a matrix phase. Group V elements-based intermetallics can lead to interesting crystalline structures that enable new concepts for anode materials, such as topotactic reaction, quasi-intercalation reaction and other interesting insertion and conversion reactions, to be designed.
Although research on the alloy-based anode materials for Li-ion battery has a long history since 1971, a breakthrough is required to bring out their full potential for Li-ion battery. Development for alloy-based anodes will remain a highly competitive field providing us with an excellent and fascinating energy source.
Read more in the review ‘Li-alloy based anode materials for Li secondary batteries’ in Chemical Society Reviews. And if you are interested in energy-related research, check out the Renewable Energy themed issue.