Jonathan C. Chen, Shengjie Zhai and Hui Zhao
Department of Mechanical Engineering, University of Nevada, Las Vegas NV, USA
The process of mixing the base and curing agent of PDMS often leads to air bubbles within the prepolymer due to its chemical reaction. The presence of air bubbles significantly decreases the strength and diaphaneity of the PDMS chip. Therefore, removing bubbles from PDMS becomes necessary and important. The traditional degasing method is at least 2 hours for a 10g PDMS mixed solution, which appears too long. A way to shorten the degasing time is in need.
Here, we develop a simple and robust method to speed up the degasing process. By periodically stopping the pump and pulling out the hose, we can remove the bubbles by forcing them to burst since the bubble cannot withstand the dramatic change in pressure, considering the large difference between the low pressure inside the vacuum and the higher atmospheric pressure outside. Using this process, we can speed up the process by around 40 minutes (10-15 gram PDMS solution) and fabricate a smooth and bubble-free PDMS sample for any purpose, especially for optical applications. In practice, this time reduction may depend on the vacuum itself and the volume of PDMS solution.
What do I need?
- SYLGARD 184 silicone elastomer base & curing agent (Dow Corning)
- 1 Disposable polystyrene weighing dish (LxWxH 86mm x 86mm x 25mm, white)
- Gast Doap 704aa compressor vacuum pump 18 HP 115 Vac
- Bel-Art vacuum chamber and plate (Interior volume 0.21 cu. ft.)
- IKA Ceramag Midi magnetic stirrer ceramic hot plate (50-1200RPM)
- 1 magnetic stirring rod octahedral 1” x 5/16”
What do I do?
- Weigh the PDMS base and curing agent (10:1) in the weighing dish.
- Mix the base and curing agent together mechanically with a magnetic stirrer. (About 1000RPM to 1200 RPM)
- Move the dish to assure that the stir bar is around all sides of the weighing dish for proper mixture for about 10-20 minutes. (Depending on amount of PDMS used)
- Place the mixed sample into the vacuum chamber, turn on the pump, and leave for about 10 minutes.
- After the 10 minutes, there should be a significant amount of air bubbles appearing on the surface. Turn off the pump, quickly pull the vacuum chamber valve out, and let outside air in. Such action changes the pressure of the vacuum chamber, eliminates most of the big surface bubbles, and pulls out the small bubbles in the solution.
- Place the hose back on and turn on the pump again.
- Repeat step 5 and step 6 until there are no more bubbles on the surface and in the solution.
- Cast the treated PDMS over the desired mold, e.g. a patterned wafer.
- Cure at 65°C for 1.5 hours.
What else should I know?
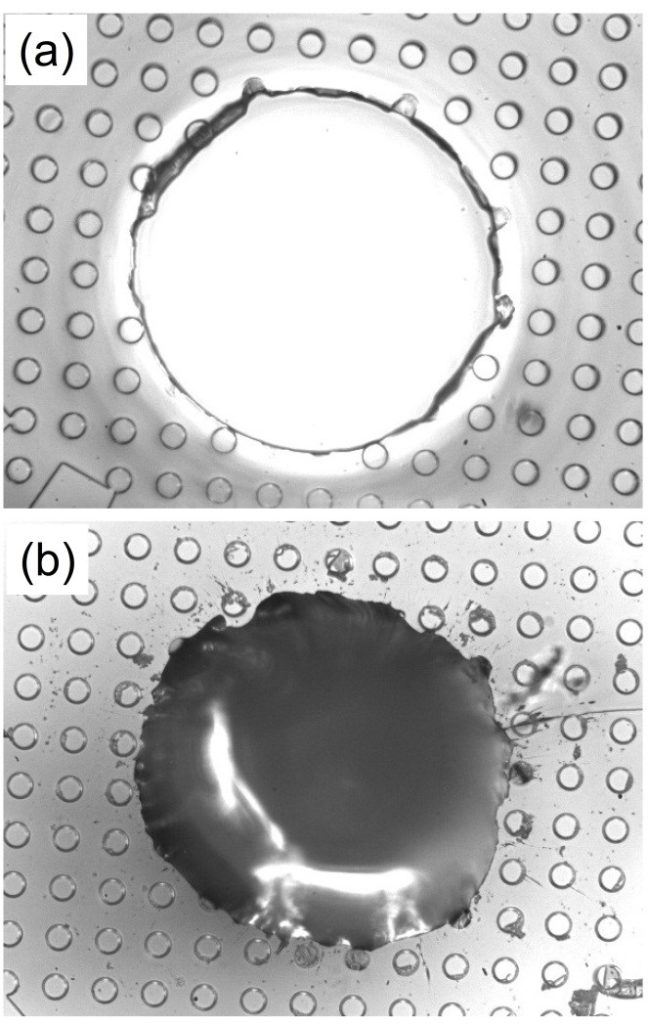
If the PDMS is used as a mold and placed in a petri dish with a microscopic glass slide, air bubbles will be significantly harder to remove due to bubbles trapped under the slide. This generally requires longer time within the vacuum and the occasional displacement of the slide to release any trapped air bubbles.
Another thing to note is that when casting the treated PDMS onto the desired mold; make sure not to pour out the mixture too fast. The slower the mixture is poured in, the less likely there will be air bubbles created during the transfer. If the sample generates air bubbles during the casting step, placing the product in the vacuum again for another 10 minutes will eliminate any unwanted bubbles.

Figure 1: Measure out a 10:1 ration of base to cure.

Figure 2: Mechanically stir the mixture.

Figure 3: Turn off the pump and use the difference in pressure to eliminate the bubbles.

Figure 4: Pour the mixture into the desired mold slowly.

Figure 5: Cure at 65°C for 1.5 hours.

Figure 6: The fabricated PDMS sample without bubbles.

Figure 7: The PDMS sample with a microscopic slide placed in.