Gabriele Pitingolo1,3, Raffaele Vecchione1,3 and Paolo A. Netti1,2,3
1 Center for Advanced Biomaterials for Healthcare, Istituto Italiano di tecnologia (IIT@CRIB), Largo Barsanti e Matteucci, 53, 80125, Naples, Italy.
2 Dipartimento di Ingegneria Chimica, dei Materiali e della Produzione Industriale D.I.C.MA.P.I, Università di Napoli Federico II, Naples 80125, Italy.
3 Centro di Ricerca Interdipartimentale sui Biomateriali (CRIB), Università di Napoli Federico II, p.le Tecchio 80, Naples, 80125, Italy
Why is this useful?
Microfluidic channels, and microstructures in general, are made by various techniques including photolithography coupled with wet etching, reactive ion etching, stamp-based techniques, such as soft lithography, hot embossing and injection molding, as well as ablation technologies like conventional machining, laser ablation and finally direct 3D printing.1 Among these techniques, PDMS soft lithography is commonly and used to replicate polymer microstructures, and particularly microchannels.2, 3
Conversely, casting a PDMS replica from a PDMS mold is challenging as both PDMS layers significantly adhere to each other and demoulding is, if at all, only possible after a careful manual cutting and peeling. A less fiddly but more elaborate approach is the passivation of the first PDMS copy by silanisation in order to reduce adhesion. Particularly, in order to prevent adhesion of the PDMS replica on the master, in a conventional process the master is treated with oxygen plasma to activate the surface and immersed for about 2 min into a silane solution (i.e., a mixture of 94% v/v isopropanol (Sigma Aldrich), 1% v/v acetic acid (Sigma Aldrich), 1% v/v Fluorolink S10 (Solvay), and 4% v/v deionized water) and then placed in an oven at 80 °C for 1 h, thus allowing a complete reaction of the master surface with the fluorinated polymer. This long and expensive process uses materials that are toxic if not removed thoroughly from the master. Recently Gitlin et al. proposed an alternative method utilising hydroxypropylmethylcellulose (HPMC) to passivate a PDMS mold4. Wilson and colleagues presented an “incubation” procedure using a 1% gelatin solution to passivate the PDMS mold5, but this method lacks the ability to control the gelatin layer thickness. Our tip shows a precise method for preparing a thin gelatin layer by spin coating technology which helps preserve the geometry of microstructures on the PDMS mold. In addition, the use of the spin coater makes controlling the gelatin thickness easier.
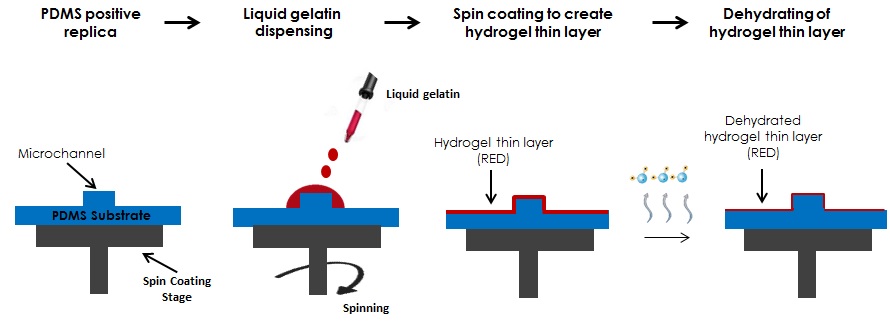
Here we propose the use of a thin hydrogel layer created with spin coating technology or other thin layer depositing techniques as a passivating material which is easy to use and less toxic than other passivating materials. In addition, this process yields hydrogel coated microstructures since gelatin remains on the replicated structures unless it is removed by peel off.
General scheme of the process
What do I need?
- Poly(methyl metacrilate) (PMMA) sheet
- Poly(dimethylsiloxane) PDMS pre-polymer
- Porcine gelatin type A
- Micromachining machine or similar
- Spin coater
- Oxygen plasma machine (optional)
- Tweezers (optional)
What do I do?
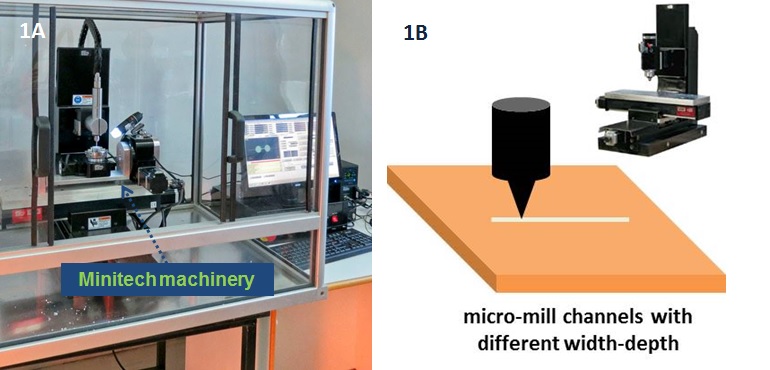
1. Mill microstructures and related inlet/outlet holes (in the case of microchannels) using a micromilling machine (Minitech CNC Mini-Mill) (fig. 1A-1B). To design a draft of the microstructures we created a layout with Draftsight (Cad Software). During micromilling, spindle speed, feed speed and plunge rate per pass were set to 12 000 rpm, 15 mm/s, and 20, respectively.
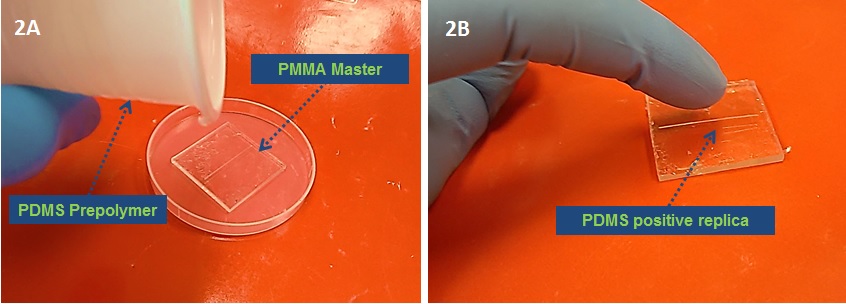
2. After micromilling, the PMMA master is ready to use. Pour liquid PDMS prepolymer (10:1) onto the master to fabricate a PDMS positive replica and cure at 80 °C for 2 h (Fig 2A-2B). The PDMS precursor is previously exposed to vacuum to eliminate air bubbles for at least 30 min.
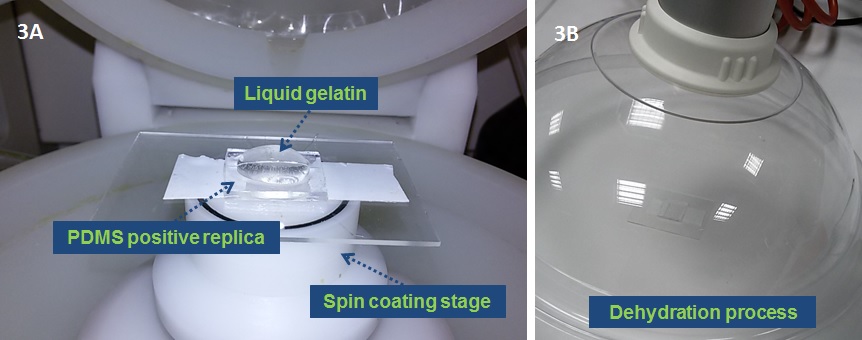
3. Place the PDMS positive replica onto a spin coating stage, deposit a small (~1 ml) droplet of liquid 10% w/v gelatin, previously degassed with nitrogen for 10 min at the center of the substrate and then spin at high speed (2000 rpm for 20 sec) (Fig. 3A). Afterwards put the system into the fridge for 20 min at 4°C, to finalize the gelling process. Dehydrate the hydrogel layer at room temperature for 5 hours under hood aspiration. (Fig. 3B) Alternatively, prepare the gelatin coating via spray deposition.
4. The Hydrogel-PDMS positive replica (HPPR) is completely dehydrated and ready to cast a new PDMS replica. IMPORTANT: only use a curing temperature below 37° C when making replicas.
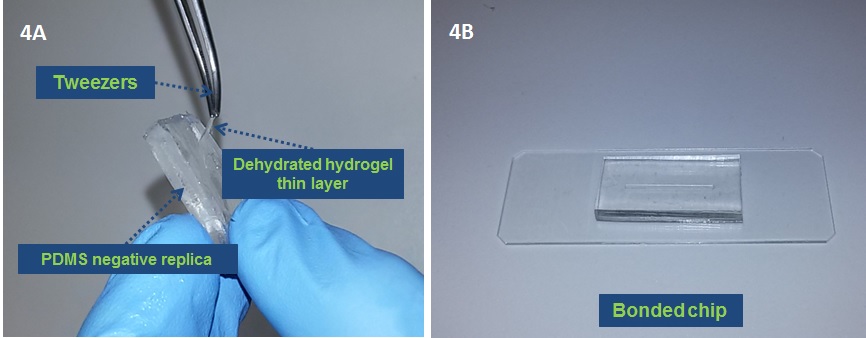
5. (Optional) After PDMS curing remove the dehydrated hydrogel layer with a tweezers from the PDMS negative replica (Fig 4A).
6. (Optional) Treat the PDMS replica with O2 plasma and bond the chip to make it ready to use (Fig 4B).
CONCLUSIONS: In this tip a double replica of PDMS was obtained by the use of an intermediate layer of gelatin. Spin coating or other thin layer deposition techniques ensure the manufacture of a very thin hydrogel layer which preserves the initial geometry of the microstructure by changing the gelatin concentration. Furthermore the dehydrated hydrogel layer ensures a biocompatible coating of the microstructures.
References
1. H. Becker and C. Gartner, Electrophoresis, 2000, 21, 12-26.
2. Y. N. Xia and G. M. Whitesides, Angewandte Chemie-International Edition, 1998, 37, 550-575.
3. S. Brittain, K. Paul, X. M. Zhao and G. Whitesides, Physics World, 1998, 11, 31-36.
4. L. Gitlin, P. Schulze and D. Belder, Lab on a Chip, 2009, 9, 3000-3002.
5. M. E. Wilson, N. Kota, Y. Kim, Y. D. Wang, D. B. Stolz, P. R. LeDuc and O. B. Ozdoganlar, Lab on a Chip, 2011, 11, 1550-1555.










