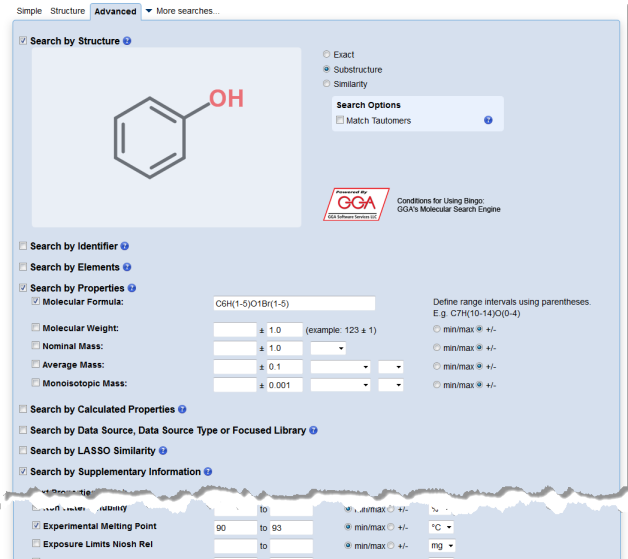
Written by Colin Batchelor.
Recently I heard someone who cycled the 1400 km from John O’Groats to Lands End, with a headwind all the way, because it looked on the map as if it was downhill and hence easier. (I am grateful to Neil Swainston of the University of Manchester for this anecdote.)
You might think that “down” on the page is unlikely to be “down” in 3D space, but there is an interesting exception to this, at least for certain interpretations of “down”.
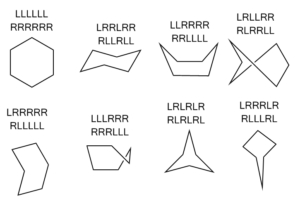
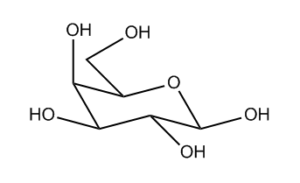
Some time ago I gave a teaser of my Sheffield talk, which is now online here and here. The mathematical meat of the talk was about redrawing sugar rings in small molecules so that they can be properly indexed by cheminformatics systems. The teaser showed a classification of hexagons so we can tell which rules to apply.
It turns out that for the hexagons we see most in practice, which are chair hexagons and Haworth hexagons, at least if the hexagon itself has its long axis roughly horizontal on the page, then if a bond points “down” on the page, when we redraw the hexagon as viewed from “above”, then the bond will still be pointing down and needs to be redrawn with a dashed bond. The same applies, mutatis mutandis, for the bonds pointing “up”.
So far, so distressingly simple. Sometimes tasks really are easier than they look. There are two more things to address, though. One is simple and involves the well-known rules for how many stereobonds you draw in any given structure (I’ve mentioned this before). The other one is tidying the molecule so that the layout algorithm doesn’t undo all your good work. This is a bit trickier and I need to look a bit more at what tools are already out there for doing this.