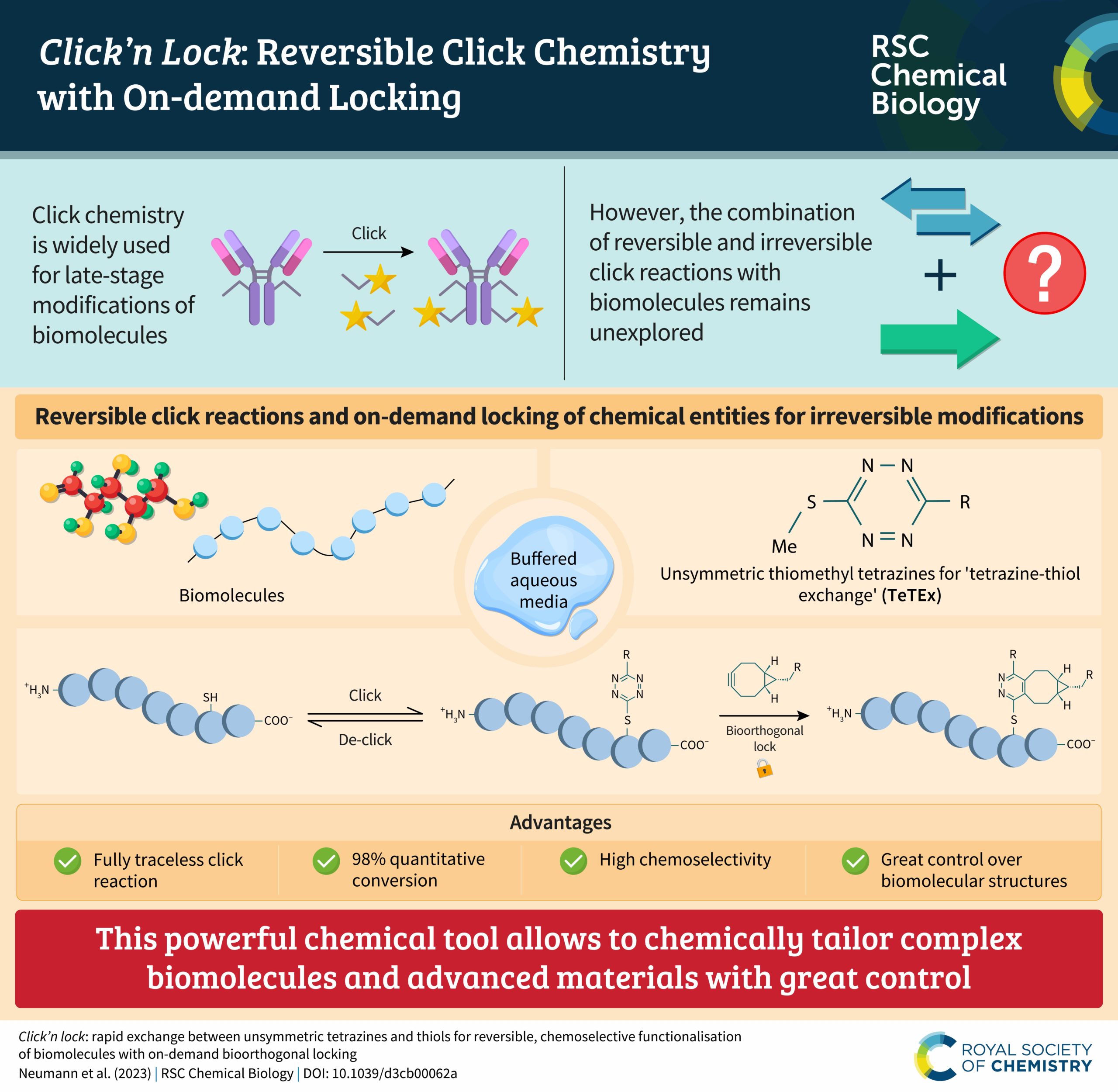
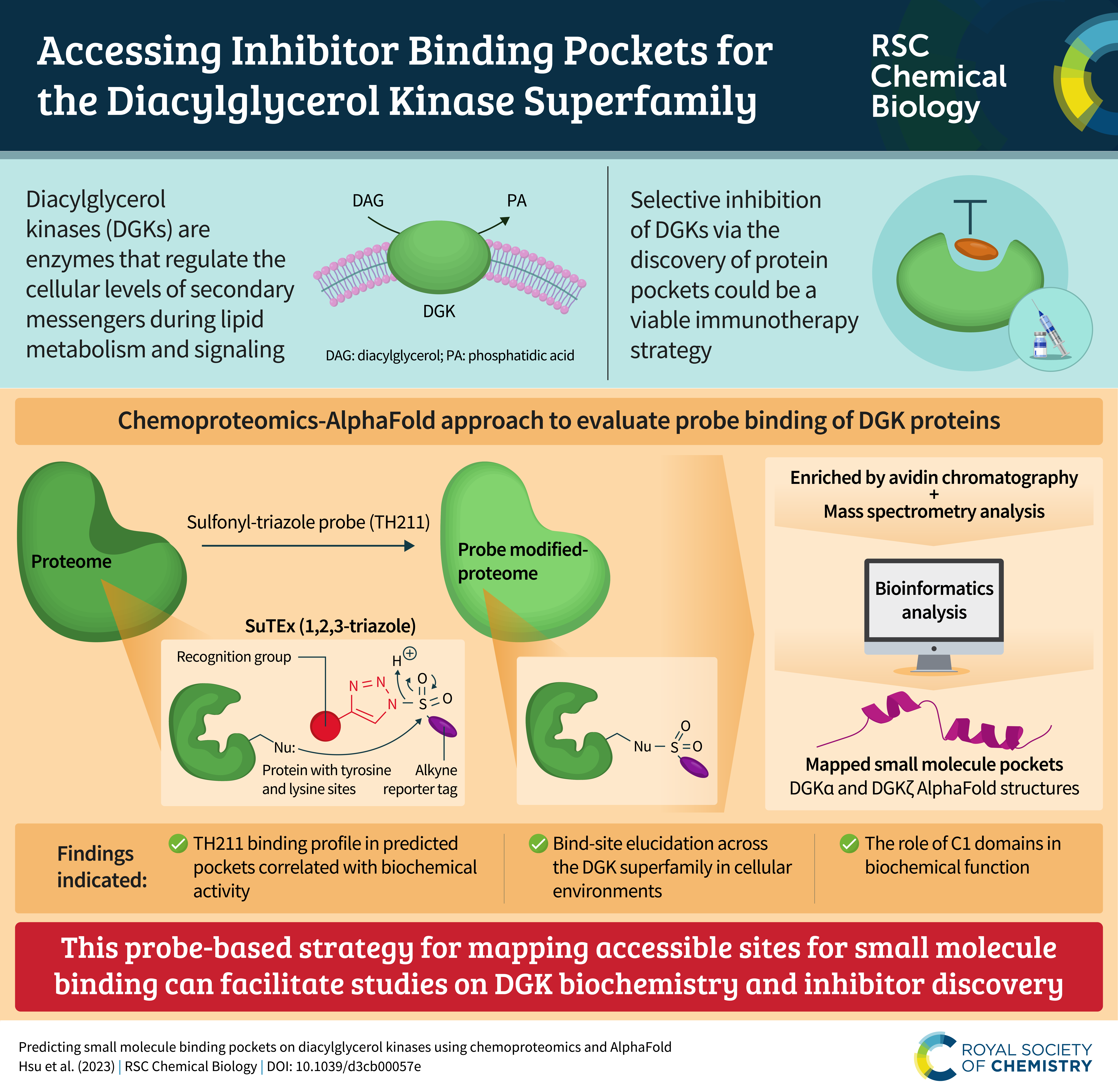
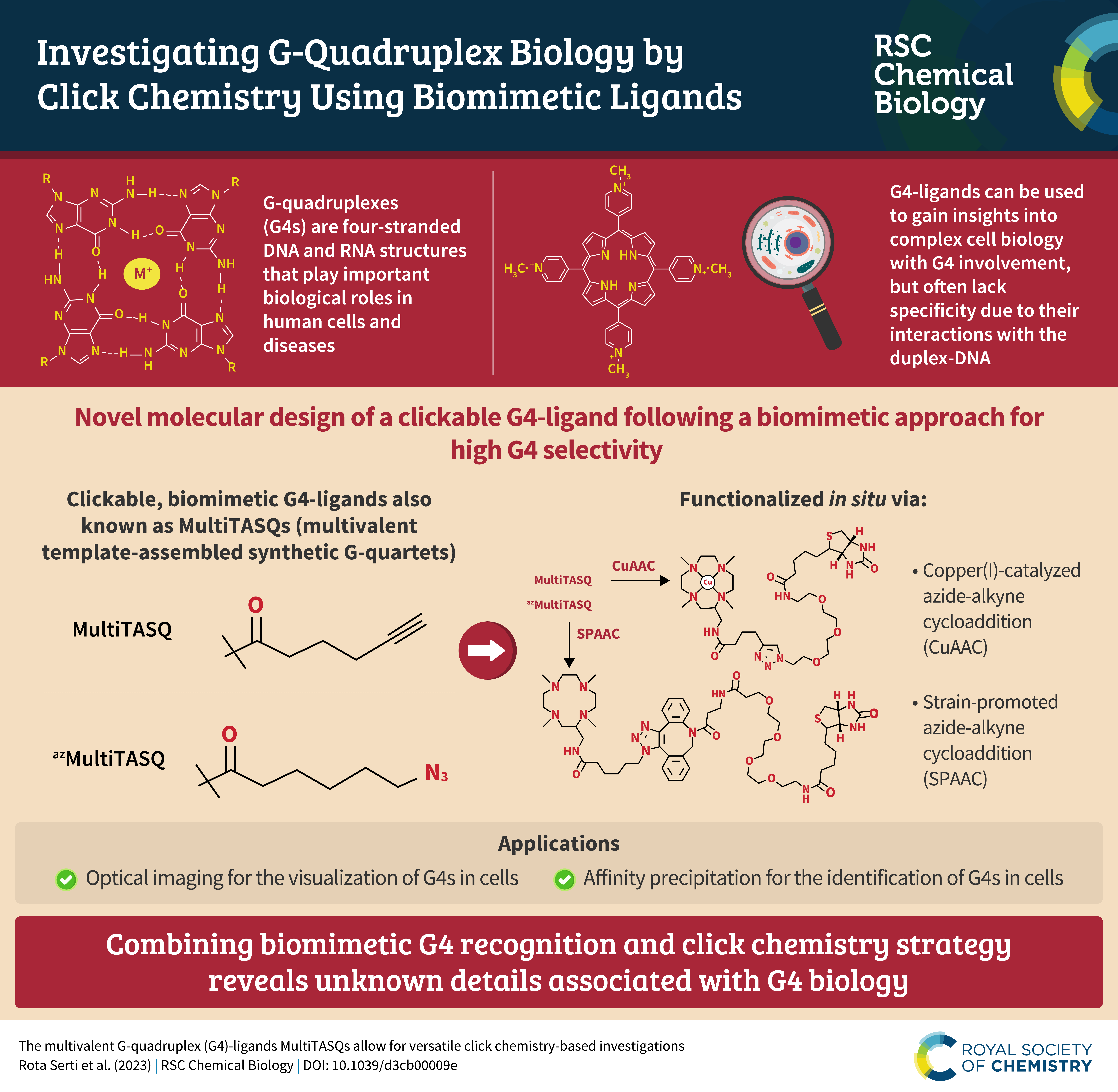
We’re pleased to announce that the latest RSC Chemical Biology Emerging Investigators collection has now been published online!

This collection highlights the work of outstanding early career researchers from across the chemical biology community. We’ve provided links to these articles and the summary Profile below. All articles in RSC Chemical Biology are open access and free to read.
If you would like to nominate a colleague or yourself as an Emerging Investigator for our next collection, please contact us for further details. Emerging Investigators must be group leaders or principal investigators in the first 10 years of their independent career.
Profile
Contributors to the 2024 RSC Chemical Biology Emerging Investigators Collection
RSC. Chem. Biol., 2025, DOI: 10.1039/D5CB90017D
Communication
Redox-neutral, metal-free tryptophan labeling of polypeptides in hexafluoroisopropanol (HFIP)
Mohammad Nuruzzaman, Brandon M. Colella, Zeinab M. Nizam, Isaac JiHoon Cho, Julia Zagorski and Jun Ohata
RSC. Chem. Biol., 2024, 5, 963–969, DOI: 10.1039/D4CB00142G
Papers
Reverse transcription as key step in RNA in vitro evolution with unnatural base pairs
Eva S. Hoffmann, Mareike C. De Pascali, Lukas Neu, Christof Domnick, Alice Soldà and Stephanie Kath-Schorr
RSC. Chem. Biol., 2024, 5, 556–566 DOI: 10.1039/D4CB00084F
Expanding the repertoire of GalNAc analogues for cell-specific bioorthogonal tagging of glycoproteins
Abdul Zafar, Sandhya Sridhar, Ganka Bineva-Todd, Anna Cioce, Nadia Abdulla, Vincent Chang, Stacy A. Malaker, David S. Hewings and Benjamin Schumann
RSC. Chem. Biol., 2024, 5, 1002–1009, DOI: 10.1039/D4CB00093E
Superoxide-responsive quinone methide precursors (QMP-SOs) to study superoxide biology by proximity labeling and chemoproteomics
Hinyuk Lai and Clive Yik-Sham Chung
RSC. Chem. Biol., 2024, 5, 924–937, DOI: 10.1039/D4CB00111G
A nanoengineered tandem nitroreductase: designing a robust prodrug-activating nanoreactor
Mariia Zmyslia, Michael J. Capper, Michael Grimmeisen, Kerstin Sartory, Benedikt Deuringer, Mohamed Abdelsalam, Kaiwei Shen, Manfred Jung, Wolfgang Sippl, Hans-Georg Koch, Laurine Kaul, Regine Süss, Jesko Köhnke and Claudia Jessen-Trefzer
RSC. Chem. Biol., 2025, 6, 21–35, DOI: 10.1039/D4CB00127C
Development of selective nanomolar cyclic peptide ligands as GBA1 enzyme stabilisers
Rebecca E. Katzy, Renier H. P. van Neer, Maria J. Ferraz, Kim Nicolai, Toby Passioura, Hiroaki Suga, Seino A. K. Jongkees and Marta Artola
RSC. Chem. Biol., 2025, 6, 563–570, DOI: 10.1039/D4CB00218K
Development of a fluorescence-based assay for RecBCD activity using Functional Data Analysis and Design of Experiments
Adam Winnifrith, Steven R. Brown, Piotr Jedryszek , Christopher Grant, Philip E. Kay, Adam M. Thomas, Jacob D. Bradbury and Thomas Lanyon-Hogg
RSC. Chem. Biol., 2025, DOI: 10.1039/D4CB00291A