As we enter the last quarter of 2025, we are excited to share our latest updates with the RSC Chemical Biology community.
Get future updates directly to your inbox with our email alerts. Sign up here.
Latest News

Our 2024 Outstanding Early Career Researcher Award goes to a team comprising Kilian Roßmann, Ramona Birke, Joshua Levitz, Ben Jones and Johannes Broichhagen, for their paper “Red and far-red cleavable fluorescent dyes for self-labelling enzyme protein tagging and interrogation of GPCR co-internalization”. Our congratulations to the winners! Find out more about the team and their paper at our blog post.
Research Spotlight
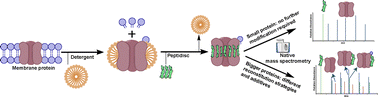
Peptidiscs offer a way to stabilise membrane proteins for mass photometry and cryo-EM in their native structure. In a recent article, Robinson, Bolla et al. have extended this to mass spectrometry, and share their insights on appropriate technique and the wide applicability of these stabilisers. Read “Native Mass spectrometry of membrane proteins reconstituted in peptidiscs” to find out more.
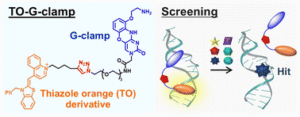
Onizuka, Nagatsugi et al. have devised new RNA-binding fluorogenic molecular probes for FID assays by combining thiazole orange derivatives with G-clamp, which were sensitive to hit compounds not found by the well-known indicator TO-PRO-1. Read their paper, “RNA-binding fluorogenic probes: G-clamp conjugated with a thiazole orange derivative for screening RNA-binding small molecules”, for more.
A new Review from Galenkap, van den Noort, and Maglia investigates the “Dynamics of single enzymes confined inside a nanopore”. Read the article to discover the promise of nanopore technology in studying single-molecule enzymology, the underlying principles, and the insights these techniques offer.
RSC Chemical Biology in the Community
We were proud to sponsor the second RSC CBIC (Chemistry Biology Interface Community) Early Career Researcher Leadership Retreat at Imperial College London, continuing our partnership with this new event. Anna Rulka, the journal’s Executive Editor, took part and presented an overview of publishing with the Royal Society of Chemistry and the support available to early career authors. Find out more about the meeting on our blog post, and in Imperial College London’s news article.

In October, Dr Rulka (centre) attended the joint ICBS & ECBS meeting ChemBioParis2025 to award poster and talk prizes sponsored by RSC Chemical Biology, meet our community, and learn from the state of the art scientific program. Our Associate Editor Zaneta Nikolovska-Coleska (second from right) also participated. Our congratulations to Hayoung Son (Seoul National University, Korea, third from left) on their Best Trainee Communications Prize, and Sebastian Hecko (TU Wien, Austria) and Yuko Hirata (University of Tokyo, Japan) on their best poster awards!

Dr Rulka was also pleased to attend the satellite 5th Young Investigator Workshop of the EuChemS Division of Chemical Biology and Chemistry in Life Sciences, at Institut Pasteur. RSC Chemical Biology proudly sponsor this event, which brings together early career researchers nominated by the EuChemS’ member National Chemical Societies.

The journal’s new Deputy Editor Alexander Whiteside (left) joined the EMBO “The epitranscriptome” workshop this October to build connections with this rapidly developing field and present prizes from RSC Chemical Biology and Molecular Omics to the authors of the best posters, Lea Pradel (EPFL, Switzerland) and Aniek Martens (Radboud University, Netherlands). Congratulations to the winners!

Collections
Our new themed collection on “Biomolecular Technologies”, Guest Edited by Prof. Sheel Dodani (The University of Texas at Dallas) and Prof. Ariel Furst (Massachusetts Institute of Technology), brings together engineered biomolecule-based technologies spanning small molecules, nucleic acids, and proteins, with applications in biocatalysis, biosensing, and synthetic biology. Read the completed collection here.
Do you have some exciting new research in nucleic acids or peptides? Consider our upcoming themed collections, currently open for submissions:
Our collection Chemical biology of nucleic acids: modifications, interactions, and therapeutic applications is open for submissions until 29 January 2026. Guest-Edited by Satoshi Obika (Osaka University), Hiroshi Abe (Nagoya University), and Michal Hocek (Czech Academy of Sciences), this collection invites research on the design and synthesis of nucleosides, nucleotides, and oligonucleotides, as well as evaluations of their biological activities both in vitro and in vivo. In addition, submissions focused on chemical biology research and drug discovery related to long DNA and RNA, and the development of new chemical biology techniques and analytical methods for nucleic acids, are also encouraged. Read the call for papers for information on how to contribute your work.
Rahul Jain (NIPER), Alex Deiters (University of Pittsburgh) Brett VanVeller (Iowa State University), and Krishna K. Sharma (Iowa State University) lead our collection on Peptide chemistry and biology: emerging technologies and translational applications. Developments in peptide modifications and new applications in PROTACs or as protein inhibitors underscore a bright future for peptide chemistry and biology. This themed collection showcases that progress, highlighting cutting-edge research from clinical and translational advances to innovative molecular designs, biological insights, and computational design approaches. Read the call for papers for information on the collection, and how to contribute your work by 29 January 2026.
Upcoming Events
Executive Editor Anna Rulka, and Associate Editor Zaneta Nikolovska-Coleska will be at this year’s Pacifichem symposium in Honolulu, Hawaii, on 15-20 December 2025. This year’s meeting includes sessions organised by our Editor-in-Chief Hiroaki Suga. We look forward to meeting you there!
We’re proud to sponsor a best poster award at the 29th Enzyme Mechanisms Conference in Carlsbad, CA, United States, January 4-8 2026.
RSC Chemical Biology is also pleased to support the 7th DNA Repair/Replication Structures & Cancer Conference in Playa Mujeres, Mexico, 1-6 February 2026.
Stay Connected
Follow us on LinkedIn and Bluesky for new articles and the latest news from RSC Chemical Biology and related journals at the Royal Society of Chemistry.