What we think of as “organic chemistry” doesn’t often focus on the structure and bonding environments of the carbon atom, unlike inorganic chemistry which often does so with heteroatoms. So I don’t think I’m going far out on a limb when I say that perhaps the key development in the structure and bonding of carbon over the last quarter century has been the isolation and use of the persistent carbene.
Arduengo carbene
A carbene is a divalent carbon atom possessing two electrons. Once thought to be unisolable, its history is recent enough that almost all important figures in its development are not only still living to this day, but still working. Ron Breslow proposed that carbenes could be isolated in 1957, Guy Bertrand isolated a liquid dicarbene in 1989 and, in 1991, Anthony Arduengo reported the first comprehensive solid-state data of the type of carbene that now bears his name (pictured above).
So why waste the words of a short post blog on historical perspective?
Because the uses of the carbene have fallen almost entirely in one direction. Primarily, the carbene has been used as a strong s 2-electron donor – attached to many transition metal atoms it forms very stable complexes, and it is relatively easy to vary its size.
Only recently have the uses of the carbene diverged.
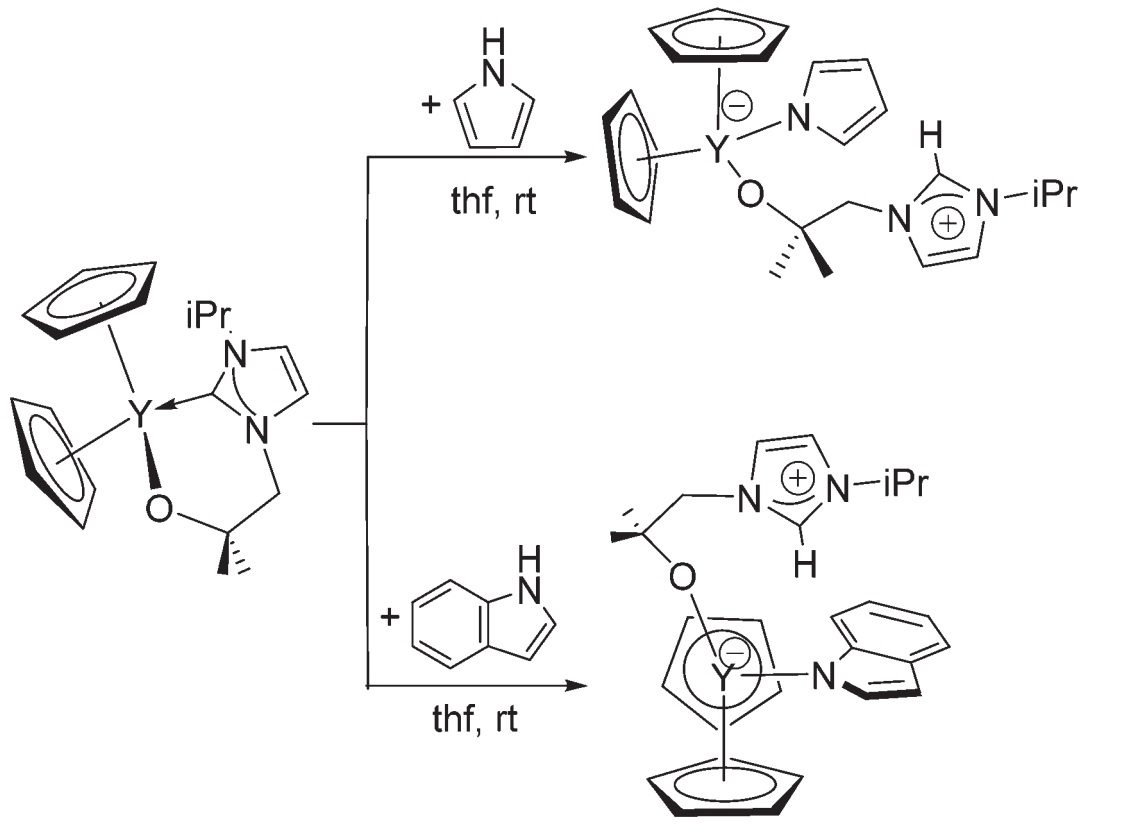
In a recent Dalton Transactions paper, Arnold, Love and co-workers present work on labile carbenes tethered to rare earth metals by an alkoxy arm. On rare earth metals – which are much harder Lewis acids than late transition metals – the soft carbene donor and hard metal acceptor are poorer matches. Thus the carbon-metal bond may break, freeing the carbene for reactivity, while the hard alkoxy arm keeps the ligand tethered to the metal.
Reactivity of tethered alkoxycarbene complexes
The authors present some examples of reactivity, including co-operative activation of pyrroles and alkynes by the carbene and metal centres, as well as outer-sphere interactions in the form of hydrogen bonding between pyrroles and the metal-bound O atom in solution. Despite these being small steps, they are nonetheless important if the carbene is to find new roles outside stabilising late transition metal centres.
Interested in finding out more? Read the full paper:
Homo- and heteroleptic alkoxycarbene f-element complexes and their reactivity towards acidic N–H and C–H bonds
Polly L. Arnold, Thomas Cadenbach, Isobel H. Marr, Andrew A. Fyfe, Nicola L. Bell, Ronan Bellabarba, Robert P. Tooze and Jason B. Love
Dalton Trans., 2014, DOI: 10.1039/C4DT01442A
 |
Ian Mallov is currently a Ph.D. student in Professor Doug Stephan’s group at the University of Toronto. His research is focused on synthesizing new Lewis-acidic compounds active in Frustrated Lewis Pair chemistry. He grew up in Truro, Nova Scotia and graduated from Dalhousie University and the University of Ottawa, and worked in chemical analysis in industry for three years before returning to grad school. |