Posted on behalf of Ian Mallov, web writer for Dalton Transactions
The water oxidation reaction is one of the most fundamental in chemistry and likely to be one of the first students are introduced to. During the reaction, water and energy are combined to give oxygen gas, protons and electrons, with the latter used to reduce the protons and yield hydrogen gas. The storage of hydrogen gas is one proposed method of storing the energy generated from renewable sources as, by burning the hydrogen and regenerating water in the process, you can release and harness the energy as needed.
In order for the water oxidation cycle to result in a net energy gain, a catalyst is needed for the oxidation process, with the majority of current systems incorporating rare or expensive metals. In their recent Dalton Transactions article, Johnsson and co-workers used small, cluster compounds made of cobalt, selenium, oxygen and chlorine to catalyze water oxidation. Two of the compounds, Co4(SeO3)3Cl2 and Co3Se4O10Cl2, were previously unreported but are closely related to the third, known compound Co5Se4O12Cl2. To synthesise the molecules, the authors heated mixtures of CoO, SeO2, and CoCl2 to 550 °C in a furnace for a number of days, with different ratios of the starting materials used to produce the different compounds. Adding each compounds to a solution of phosphate buffer and Ru(bpy)3(PF6)3 led to the evolution of oxygen gas, which, by further 18O labelling experiments, was confirmed to occur due to water oxidation.
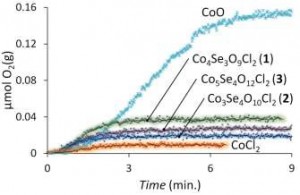
Catalytic oxygen evolution by cobalt catalysts
Analysis of a catalyst intended to facilitate sustainable energy storage should spur discussion of the environmental impact of making the catalyst. The large energy input to heat the materials is a drawback. But, if synthesized in good yield in solid-state (solventless) reactions, as done here, the reactions would score well on the E-factor scale, a metric measuring waste produced per mass of product that gives a more complete indication of material efficiency than the obsolete atom economy principle.
Though the catalytic activity proved wanting, the simple compounds and syntheses here present an interesting strategy towards useful water oxidation catalysis.
Find out more and download the article now:
Cobalt selenium oxohalides: Catalysts for Water Oxidation
Dalton Trans., 2013, DOI: 10.1039/C3DT53452A
 Ian Mallov is currently a Ph.D. student in Professor Doug Stephan’s group at the University of Toronto. His research is focused on synthesizing new Lewis-acidic compounds active in Frustrated Lewis Pair chemistry. He grew up in Truro, Nova Scotia and graduated from Dalhousie University and the University of Ottawa, and worked in chemical analysis in industry for three years before returning to grad school.
Ian Mallov is currently a Ph.D. student in Professor Doug Stephan’s group at the University of Toronto. His research is focused on synthesizing new Lewis-acidic compounds active in Frustrated Lewis Pair chemistry. He grew up in Truro, Nova Scotia and graduated from Dalhousie University and the University of Ottawa, and worked in chemical analysis in industry for three years before returning to grad school.










