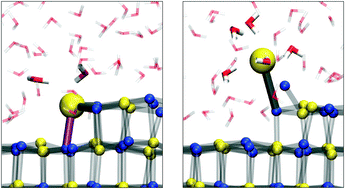
Michaelides et al. have investigated how NaCl dissolves in water using ab initio modelling of the system. The process involves several steps and begins with the loss of Cl– ions. An improved understanding of the dissolution process of salt could be applied to many environmental and atmospheric chemistry problems.
This interesting research is also highlighted on the website of University College London’s Chemistry Department.
Read the full communication:
Initial stages of salt crystal dissolution determined with ab initio molecular dynamics
Li-Min Liu, Alessandro Laio and Angelos Michaelides
Phys. Chem. Chem. Phys., 2011, 13, 13162-13166
DOI: 10.1039/C1CP21077G