Saifullah Loneab* I. W. Cheongb and S. T. Thoroddsena
aDivision of physical Sciences and Engineering, King Abdullah University of Science & Technology, (KAUST), Thuwal, 23955-6900, Saudi Arabia.
bInstitute of Advanced Energy Technology, Kyungpook National University, Daegu, South Korea,
Phone: +821053165673, Office: +82-53-950-7590, FAX: +82-53-950-6594. Email: saifullah.lone@gmail.com, inwoocheong@gmail.com, and sigurdur.thoroddsen@kaust.edu.sa
Why is it Useful?
The subject of droplet microfluidics has grown in importance among researchers in chemistry, physics and biology, hence it has found applications in drug delivery, encapsulation, single-cell analysis, pickering-emulsion and phase-separation. For generating monodisperse droplets, various methods have been employed in constructing microfluidic devices. Emulsions with a coefficient of variation ≤ 5% have been previously reported in T-junction, flow focusing, co-axial, as well as other types of microfluidic devices. Microdroplets with ≤100 µm size offer attractive applications in industry and biology. Small channel-diameters attained by clean-room soft lithography is the most precise technique for fabricating microfluidic devices.1, 2 This technique is widely used to make master molds for PDMS-based devices.3 However, regarding the cost and complexity, it is difficult to install clean-room soft lithography in financially challenged countries and laboratories. Therefore, the cost and special clean-room training restricts its wide-spread application. To develop low cost robust technologies; inkjet printing, controlled numerical machining, xurography or razor-writing, printed circuit technology, print-and-peel (PAP) microfabrication and 3-D printing have been tested to fabricate microfluidic devices without clean-room technology. However, creating droplets under 100 µm size ranges by non-cleanroom technologies is challenging and open for upgradation. Recently, a rapid prototyping technique for microfluidics has been reported by employing laser-patterned tape4 This technique relies on computer-controlled CO2 laser beam. This work was further simplified by manual razor patterned tape-based prototyping for patterning mammalian cells.5 Building on this prototyping concept, we extended the idea to produce monodisperse droplets under 100 µm size rages by overlapping the razor patterned tape strips (at right angles) on a flat glass surface. The production of monodisperse emulsion under 100 µm size ranges are greatly useful in pharmaceutical and cosmetic industries. Hence, our approach may well serve as one of the simplest approaches to fabricate droplet microfluidic generators.
What do I need?
- One-sided adhesive tape (Temflex 1500 electrical, thickness 150 µm)
- Flat glass slides, such as a microscope slide
- 30cm stainless steel metal ruler
- Sharp razor blade
- Uncured mixture of PDMS base and curing agent (10:1 w/w)
- Oxygen plasma
- Oven or hot plate
- A microfluidic PDMS puncher for drilling holes
- Deionized water (D.I. water) and 20 cSt and 10 cSt silicone oil
What do I do?
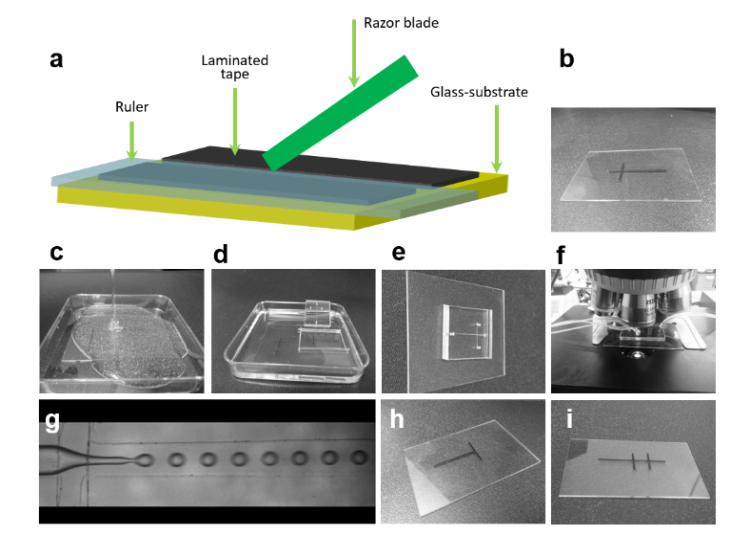
Figure 1 outlines the prototyping procedure. Prototyping begins by attaching adhesive tape on a flat glass substrate. With a sharp razor-blade, the tape is cut into fine parallel strips. The thickness of the tape (150 µm) determines the depth of the microchannel, but this can be increased by attaching multiple layers of tape on top of each other. Next the tape is removed from the regions outside the fine strips.
To construct a cross-junction, one strip of the tape is lifted and horizontally placed on top of another at an angle of 90ᴼ. The junction is pressed gently to ensure the strips are well attached. These adhering strips of tape serve as a master for PDMS-based replica casting.
A mixture of PDMS silicone elastomer base and a curing agent (in 10:1 ratio) is poured on top of the master within a plastic petri dish. The mixture is degassed under vacuum for 1 h and cured for 4 hrs at 65°C. Cured PDMS replica is then cut and peeled-off from the master. The master can be used repeatedly to fabricate multiple copies of the PDMS replica by following the afore-mentioned steps. Inlet and outlet holes are drilled through PDMS replica, which is then bonded on a glass substrate, after both replica and glass has been exposed to oxygen plasma. Figure 1(g) shows the resulting PDMS-device for generating monodisperse water-in-oil (W/O) emulsion. The technique is easily extended to fabricate T-junction or double T-junction prototypes (Figure 1h and i).
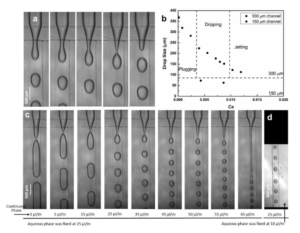
Figure 2(a), demonstrates the droplet formation at a cross junction in our tape-based microfluidic device, with a channel width and depth of 300 µm and 150 µm respectively. In Figure 2(c), we kept the flow rate of aqueous phase fixed at 25 µl/min, while systematically increasing the flow-rate of outer continuous oil-phase (20 cSt silicone oil). As the outer flow-rate is increased, the regime is found to shift from dripping at lower flow-rate to jetting at higher flow-rate (Figure 2(c)). For lowest flow-rate, the aqueous-phase breaks into elongated plugs, while at higher flow-rates regular drops are pinched off. Various factors affect the size of the droplets, but it is primarily determined by competition between viscous stress in the continuous phase Fv~ µU/d which tends to rip off the drop and the interfacial tension Fs ~ s/d which try to keep the drop attached. Here µ is the dynamic viscosity of the outer phase and U is its velocity; while s is the interfacial tension between the water and the oil, s=0.040 N/m. For small channels, the characteristic length-scale d is the same for the two forces and it therefore drops out of the balance and when Fv~ Fs then the non-dimensional capillary number Ca=µU/s characterizes their relative strength. Figure 2b shows the droplet-size as a function of Ca. When the flow-rate of oil-phase reaches 65 µl/min, the size of the droplets reaches ~100 µm and the droplet breakup occurs at a large distance from the cross-junction. Figure 2(d) shows drop formation with a channel width of 150 µm and a channel depth of 150 µm. In this case, the droplet size reaches down to ~73 µm, when the oil-phase (10 cSt) is flowing at 25 µl/min and aqueous-phase at 10 µl/min.
Acknowledgement: This work was jointly funded by King Abdullah university of Science & Technology (KAUST), Thuwal, Saudi Arabia , and the Ministry of Trade, Industry and Energy, Korea (Grants No. 10067082 and 10070241).
Reference
[1] Qin, D.; Xia, Y.; Whitesides, G. M. Rapid prototyping of complex structures with feature sizes larger than 20 μm. Adv. Mater. 1996, 8, 917-919.
[2] Xia, Y.; Whitesides, G. M. Soft Lithography. Angew. Chem. Int. Ed. 1998, 37 550- 575.
[3] Duffy, D. C.; McDonald, J. C.; Schueller, O. J.; Whitesides, G. M. Rapid Prototyping of Microfluidic Systems in Poly (dimethyl siloxane). Anal. Chem., 1998, 70, 4974–4984.
[4] Luo, L. W.; Teo, C. Y T.; Ong, W. L.; Tang, K. C.; Cheow, L. F.; Yobas, L. Rapid prototyping of microfluidic systems using a laser-patterned tape J. Micromech. Microeng. 2007, 17 N107–N111
[5] Anil, B. S.; Ali, H.; Cheul, H. C.; and Raquel, P-C. Adhesive-tape soft lithography for patterning mammalian cells: application to wound-healing assays. BioTechniques, 2012, 53 315–318.