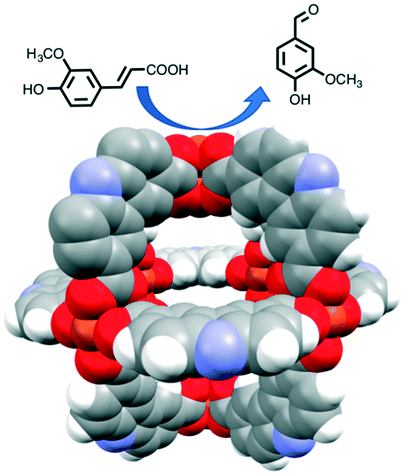
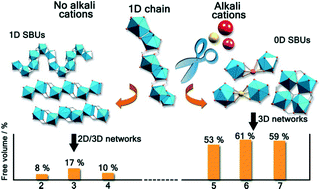
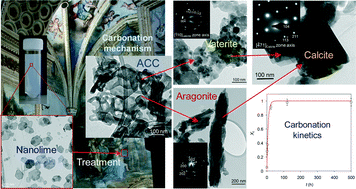
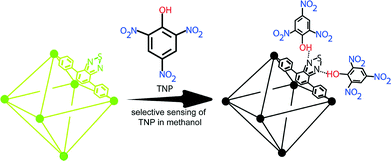
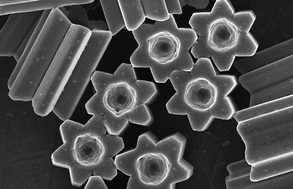
Inorganic scintillators have desirable properties for the detection of ionising radiation, with potential applications including in nuclear instrumentation. Scintillators are materials which produce light when they interact with ionising radiation allowing the radiation to be detected and quantified. However, the methods used to prepare inorganic scintillators are a limitation to the development of further uses. Growing crystals from the melt provides only a limited range of materials and, while glasses or glass-ceramics allow a wider range, these materials typically have a low light intensity. Polycrystalline ceramics lie between single crystals and glasses in terms of properties.
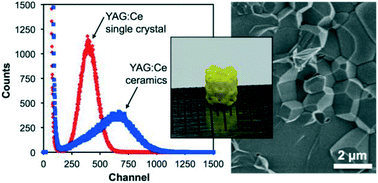
In a recent paper, Dosovitskiy et al. demonstrate the preparation of the scintillator YAG:Ce (yttrium aluminium garnet doped with cerium activator) as a polycrystalline ceramic using 3D-printing. This was achieved for Y2.97Ce0.03Al5O12 by co-precipitation followed by heating at 900 oC. A slurry of the resulting powder was then used to 3D-print green bodies using stereolithography. A so-called green body contains the material of interest along with a binder, the latter being subsequently removed by heating.
After debinding and sintering at 1600 oC , the properties of the resulting ceramic material were compared with those of the corresponding YAG:Ce single crystals. The ceramic showed a scintillation light yield more than 60% higher than that of the single crystals, when using 5.5 MeV α-particles.
Higher activator concentrations can be achieved using this preparation method than in single crystals and this is beneficial to the light yield. 3D printing allows the production of shapes not available by other methods, free of defects and larger than 1 micrometer. One possible application of this is in the production of luminescent materials for LED lighting devices.
For more information, see the full paper at:
First 3D-printed complex inorganic polycrystalline scintillator
A. Dosovitskiy, P. V. Karpyuk, P. V. Evdokimov, D. E. Kuznetsova, V. A. Mechinsky, A. E. Borisevich, A. A. Fedorov, V. I. Putlayev, A. E. Dosovitskiy, M. V. Korjik
DOI:10.1039/C7CE00541E
___________________________________________________________________________________________________
 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She published a book, ‘Molecules, Medicines and Mischief’, in 2014, on some of the chemicals found in plants and is currently working on a follow-up.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She published a book, ‘Molecules, Medicines and Mischief’, in 2014, on some of the chemicals found in plants and is currently working on a follow-up.











 This work suggests some of the factors involved in the formation of curved fractal structures and also shows that interest in these extends beyond the scientific and into the realm of fashion design.
This work suggests some of the factors involved in the formation of curved fractal structures and also shows that interest in these extends beyond the scientific and into the realm of fashion design.