A new paper by Yu-Bin Dong et al reports a metal-organic framework (MOF) which not only reversibly adsorbs two different classes of volatile organic compounds (VOCs), but within each class also shows selectivity allowing separation of chemically similar examples.
VOCs are widely used in the chemical industry but their release into the environment is undesirable, as they can cause damage both to the environment and to human health. Porous materials such as MOFs are attractive as potential adsorbents of VOCs and could also allow separation of examples with similar boiling points, which proves difficult by other methods such as distillation.
The new MOF consists of ditriazole-N-butylcarbazole ligands bridging Cu ions, forming a framework with square-like pores. The ligand butyl groups point into the pores, making them hydrophobic. The adsorption of VOCs classified as chlorocarbons (e.g. CH2Cl2) and aromatic solvents such as benzene and toluene were shown to be fully reversible under ambient conditions.
Tests on the selectivity towards chlorocarbons showed that CHCl3 was adsorbed in preference to CH2Cl2 from a mixture. This suggests that, unusually, selectivity may be based on the polarity of the molecules rather than the size – CHCl3 is larger but less polar than CH2Cl2.
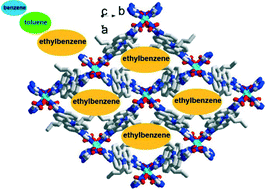
In the case of the aromatic molecules, it proved possible to separate benzene and toluene from mixtures of toluene/ethylbenzene/xylene and ethylbenzene/xylene, respectively. In both these systems, the larger molecules are adsorbed in preference to the smaller (benzene or toluene, respectively) – see diagram below.
Rather than being determined by the size or polarity of the solvent molecules, this can be explained if the hydrophobic properties are considered. More hydrophobic molecules, such as ethylbenzene, are preferentially adsorbed into the hydrophobic pores present in the MOF, where they form favourable hydrophobic interactions.
The MOF reported here adds to the range available that show specific properties with regards to adsorption and separation of VOCs, unusually demonstrating specificity related to both the polarities and hydrophobicity of the VOCs.
For more information read the full paper at:
Fan Yang, Qi-Kui Liu, Jian-Ping Ma, Yan-An Li, Ke-Xin Wang and Yu-Bin Dong
DOI: 10.1039/C5CE00547G
 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.