Successful use of pharmaceutical drugs depends on their delivery and controlled release so that their bioactivity can be harnessed. This can mediate poor solubility, degradation and other properties of the drug which might otherwise be problematic. One way to control delivery is to load the drug into a container which allows the compound to be transported to the desired location, to then be released over a suitable time period. The behaviour of the container is dependent on both the size and the shape, so simple and reliable fabrication techniques are required.
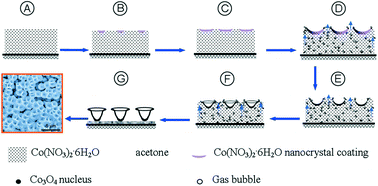
In a recent CrystEngComm article, scientists from China show how such containers can be made which are shaped like lotus leaves and are nano/microsized. The Co3O4 nano/microcontainers can be easily prepared from Co(NO3)2.6H2O by evaporation of the acetone solvent followed by calcining (i.e. heating at below the melting point). In this process, shown in the diagram below, the large amount of gas bubbles produced are key to determining the shape of the containers, with no other shape-directing agents required. The size and density of the nano/microlotus-leaf arrays can be controlled by variation of the evaporation time and temperature.
The research team used fluorescein isothiocyanate (FITC) as a model drug to study the controlled drug delivery from the nano/microlotus-leaf arrays. They found that it could be loaded and released more effectively than for comparable Co3O4 microspheres and showed that cells which were treated with the arrays retained over 80% viability even at high concentration — indicating that these microcontainers are a safe delivery vehicle of active compounds to cells.
For more details, see the paper: