
Dan Shechtman copyright Technion
Professor Dan Shechtman holds the Philips Tobias chair of Materials Science at Technion – Israel Institute of Technology. Prof. Shechtman was an NRC fellow at the aerospace Research Laboratories at Wright Patterson AFB, Ohio, where he studied for three years the microstructure and physical metallurgy of titanium aluminides. In 1975 he joined the department of materials engineering at Technion. In 1981-1983 he was on sabbatical at the Johns Hopkins University, where he studied rapidly solidified aluminum transition metal alloys (joint program with NBS). During this study he discovered the Icosahedral Phase which opened the new field of quasiperiodic crystals. In 1992-1994 he was on sabbatical at NIST, where he studied the effect of the defect structure of CVD diamond on its growth and properties. For the past 6 years he has also been a part time faculty member of Iowa State University. He recently won the Nobel Prize in Chemistry 2011 for his discovery of quasicrystals.
What achievement are you most proud of?
The whole thing. I have opened a door to something new in crystallography, and many crystallographers came in through it, resulting in a paradigm shift. Many believed, many did not, and so it was a battle of minds for ten years.
In 1982, I was alone, and couldn’t explain my results. In 1984, I returned to Technion, where my colleague Ilan Blech was the first to believe in my findings, and helped by elucidating the structure and building a model to explain this phenomenon. We submitted this in 1984 to the Journal of Applied Physics, but it was rejected, and we finally managed to publish it in Metallurgical and Materials Transactions more than half a year later. In the meantime, John Cahn (my colleague at NIST) and the French crystallographer Denis Gratias became involved with the project, and we submitted a short paper to Physical Review Letters based on my original results from day one. In the period that followed, many scientists accepted quasicrystals, but there were still many people who rejected the idea, including the International Union of Crystallography (IUCr). They wanted single crystal X-ray diffraction results to definitively confirm the existence of quasicrystals.
Between 1984 and 1987 many attempts were made to grow crystals big enough for single crystal X-ray diffraction, and two groups in Japan and France achieved it. I presented these results at the 14th IUCr Congress in Perth, Australia, and the crystallographic community finally said ‘OK Danny, now you are talking!’ and they established a committee to redefine crystals. This was very meaningful, as it demonstrated that the community could be open to new discoveries.
What drove you to stand by your results, even though you knew many people would challenge them?
Most of the people in my close environment who knew didn’t believe and were very negative about my findings, and some were even negative towards me. Some of my colleagues at NIST, where I was on sabbatical, were more subjective. My host John Cahn told me: ‘Danny, this material is telling us something, and I challenge you to find out what it is.’
I am my own worst critic. I tried everything necessary in order to convince myself that I knew what it was not. No one had a better explanation. I remember the discovery date well, April 8 1982.
Electron diffraction played a fundamental role in the discovery of quasicrystals, and it is still a growing field. What are your thoughts on electron diffraction?
Electron diffraction is a wonderful tool, and nowdays it can be a wonderful crystallographic tool. Before electron diffraction was not as precise, but now using convergent beam electron diffraction we can determine with precision the structure of tiny crystals. Electron diffraction is the tool for discovery.
What projects are you working on at the moment?
I am looking at a range of materials, such as the B2 materials which are intermetallics. There are B2 materials which are very brittle, but we are working on some which are very ductile. We are now working mostly with Mg alloys for various applications, such as biodegradable and biocompatible implants and as antibacterial materials to fight bacterial infections.
What will be the next big breakthrough?
Nobody knows! Great discoveries are stumbled on. If you are clever enough you will work hard on a problem and elucidate the answer.
Do you have any advice for young scientists?
Be an expert in something, regardless of what it is. I was good at electron microscopy, but you can be good at X-ray diffraction, synthetic chemistry etc. Read everything, familiarise yourself with the instrumentation and methodology so that when you see something different, you will realise and know that it is different, rather than thinking it is an anomaly or an error.
Find out more about Dan Shechtman on his webpage at Technion Institute of Technology. You might also be interested in reading more in my recent blog on Professor Shechtman’s Nobel Prize.
Why not check out Chemistry World’s recent story on this year’s award too!
 Copper sulfide cages wholly exposed with nanotwinned building blocks
Copper sulfide cages wholly exposed with nanotwinned building blocks 












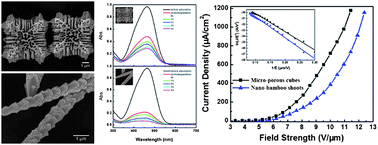 In this CrystEngComm HOT Article, two kinds of micro/nano-sized single-crystalline cuprous oxide (Cu2O) crystals with novel porous cubic or hierarchical rod-like morphologies were successfully synthesized via a facile ethanol-assisted double-solvothermal method. It was found that the addition of ethanol in precursor solution is critical for the formation of hierarchical rod-like structures (nano-bamboo shoots). Their growth process and shape evolution together with their optical properties and field emission have also been reported.
In this CrystEngComm HOT Article, two kinds of micro/nano-sized single-crystalline cuprous oxide (Cu2O) crystals with novel porous cubic or hierarchical rod-like morphologies were successfully synthesized via a facile ethanol-assisted double-solvothermal method. It was found that the addition of ethanol in precursor solution is critical for the formation of hierarchical rod-like structures (nano-bamboo shoots). Their growth process and shape evolution together with their optical properties and field emission have also been reported.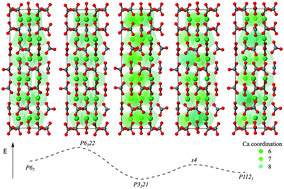 Vaterite is one of the three crystalline polymorphs of calcium carbonate, and plays a significant role in biomineralisation, either as an intermediate formed from amorphous calcium carbonate prior to transformation to aragonite or calcite, or can be utilized by organisms in its own right.
Vaterite is one of the three crystalline polymorphs of calcium carbonate, and plays a significant role in biomineralisation, either as an intermediate formed from amorphous calcium carbonate prior to transformation to aragonite or calcite, or can be utilized by organisms in its own right.