We are delighted to share the latest selections in the Molecular Omics Editor’s collection. This is a showcase of some of the best articles published in our journal, hand selected by our Associate Editors and Editorial Board members.

This selection is from our Associate Editor Professor Hyungwon Choi. Hyungwon is an Associate Professor in the Department of Medicine at the National University of Singapore.
He and his team have actively developed computational and statistical solutions for the analysis of high-throughput molecular data and integration of heterogeneous multi-omics data. His main research topics include network-driven integration of multi-omics data, protein-centric analysis of genomic and transcriptomic data in large-scale clinical studies, and bioinformatics pipeline development for mass spectrometry data extraction in metabolomics and lipidomics.
Professor Choi has highlighted some of their favourite recent articles below:
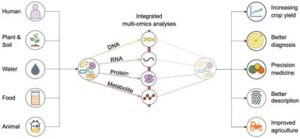 Integrated multi-omics analyses of microbial communities: a review of the current state and future directions
Integrated multi-omics analyses of microbial communities: a review of the current state and future directions
Muzaffer Arikan and Thilo Muth
Mol. Omics, 2023, 19, 607-623
Hyungwon’s comments
“Arikan and Muth provide a timely review of the current state in the use of high-throughput omics technologies in microbial community analysis. The authors describe the emerging landscape towards comprehensive, integrated multi-omic analysis and the bioinformatic tools enabling the objective. The article offers an insightful map of complex data processing workflows and recounts significant challenges in integrating heterogeneous, complex data to generate meaningful information.”
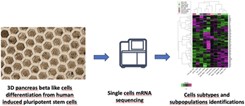 Generation of β-like cell subtypes from differentiated human induced pluripotent stem cells in 3D spheroids
Generation of β-like cell subtypes from differentiated human induced pluripotent stem cells in 3D spheroids
Lisa Morisseau, Fumiya Tokito, Stéphane Poulain, Valerie Plaisance, Valerie Pawlowski, Soo Hyeon Kim, Cécile Legallais, Rachid Jellali, Yasuyuki Sakai, Amar Abderrahmani and Eric Leclerc
Mol. Omics, 2023, 19, 810-822
Hyungwon’s comments
“Morisseau et al. previously derived human pancreatic beta cells from hiPSCs in 3D spheroids that are functionally akin to beta cells. In this paper, they expanded on the previous work to characterize cell population diversity using single cell transcriptomics analysis. Their knowledge-driven interpretation of the data delineates the composition of beta-like cell subtypes including bi-hormonal cells and potential endocrine progenitors. The work nicely showcases the potential of the differentiation protocol in physiological conditions relevant to diabetes and the power of single cell gene expression analysis.”
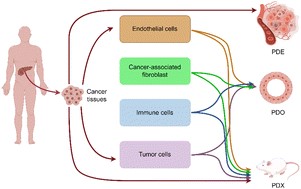 Pancreatic cancer environment: from patient-derived models to single-cell omics
Pancreatic cancer environment: from patient-derived models to single-cell omics
Ao Gu, Jiatong Li, Shimei Qiu, Shenglin Hao, Zhu-Ying Yue, Shuyang Zhai, Meng-Yao Li and Yingbin Liu
Mol. Omics, 2024, 20, 220-233
Hyungwon’s comments
“Gu et al. review the advantages and disadvantages of patient-derived models such as xenografts, organoids and explants over conventional cell cultures as experimental models for characterizing pancreatic cancer microenvironment. The authors highlight the importance of patient-derived models in preserving realistic tumor heterogeneity and complexity. In this context, they show how single cell (or spatially resolved) transcriptomics and epigenomics analysis, and potentially multi-omic approaches can decipher key biological signals in all three types of models.”
Enjoyed these articles? Check out our latest publications and if you want the chance to be part of the next edition of our Editor’s collection, submit your research here.