HOT article from FD154!
The recent Faraday Discussion 154 on Ionic Liquids took place at Queen’s University, Belfast.
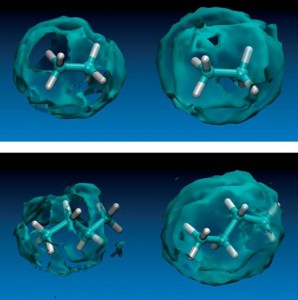
Margarida Costa Gomes presented her group’s work on the solubility and thermodynamics of solvation of ethane and n-butane in various ionic liquids. The authors found that the alkanes were more soluble in ILs with longer alkyl chains and observed preferential solvation in the non-polar part of the ILs.
Read the full HOT Faraday Discussion article today:
Using ethane and butane as probes to the molecular structure of 1-alkyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide ionic liquids
Margarida F. Costa Gomes, Laure Pison, Alfonso S. Pensado and Agilio A. H. Pádua
DOI: 10.1039/C1FD00074H