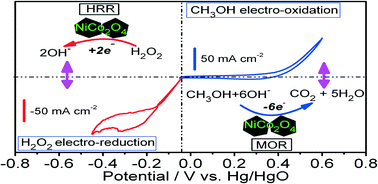
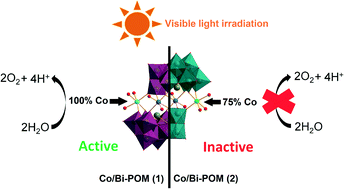
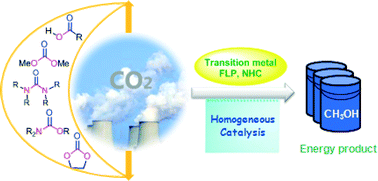
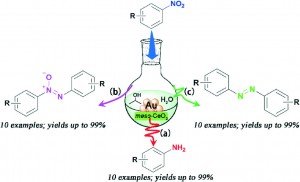
Ever heard of a customizable catalyst? A catalyst with a unique shape? A catalyst which with a change in size can provide different activity? If not, then here is a catalyst- an easy to prepare Pd nanocube which you can customize as per the activity desired: racemic or enantioselective hydrogenation of α,β-unsaturated carboxylic acids.
The enantioselective hydrogenation of aliphatic α,β-unsaturated carboxylic acids faces the obstacle of lower enantioselectivities as the aliphatic substituent is not armed to curb the inevitable isomerization of the double bonds in the structure. High enantioselectivities have been observed in cases of aryl substituted α,β-unsaturated carboxylic acids owing to the stabilizing effect of the aryl substituent at the β-position. With no effective solutions up-to-date, the researchers at Chinese Academy of Sciences tried to find the answer to this problem in the world of micromeretics and morphology.
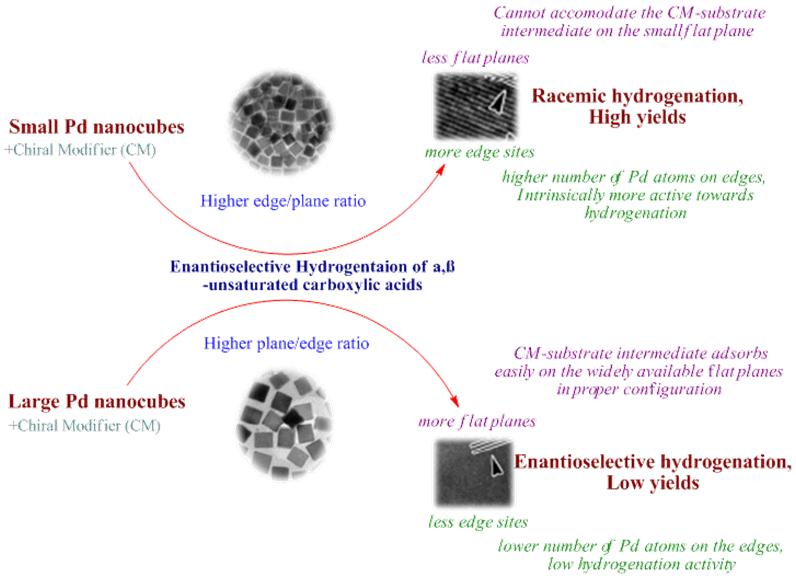
Considering the previous instances where the sizes and shape of the catalysts did play a role in the enantioselectivity, the researchers tried to capitalize on this and were indeed rewarded with fruitful results. They decided to study the effects of shape and size, by preparing both cubic and spherical Pd nanoparticles as catalysts for the enantioselective hydrogenation of unsaturated carboxylic acids. The studies conducted by the Shen group clearly indicate that the Pd nanocubes have a upperhand, as they provide good enantioselectivities as compared to the spherical nanoparticles. They also found that the Pd nanocubes of larger size provided with excellent enantioselecctivities as compared to the smaller nanocubes. The dynamics of this can be explained by the fact that larger nanocubes, which have more flat sites, can easily accommodate the chiral modifier (like cinchonidine) on its surface along with the substrate, thus resulting in higher enantioselectivities. Meanwhile, the smaller nanocubes provided higher yields, as they are equipped with more edge sites, which accelerates the process of hydrogenation. The present study provides with a customizable formula with both small and large nanocubes put to different use.
| Activity desired |
Pd Nanocubes customized to |
| Racemic Hydrogenation at High yields |
Small size |
| Enantioselective Hydrogenation at Lower yields |
Large size |
Thus, the present paper brings forward the fresh concept of customized Pd nanocubes, which can be an effective weapon in the armory of catalysts for enantioselective hydrogenation of α,β-unsaturated carboxylic acids.
Customizable Palladium Nanocubes for Racemic/Enantioselective hydrogenation
To read more, follow the link below:
Enantioselective hydrogenation of α,β-unsaturated carboxylic acids on Pd nanocubes
Chunhui Chen, Ensheng Zhan, Na Ta, Yong Li and Wenjie Shen
Catal. Sci. Technol., 2013, Advance Article
DOI: 10.1039/C3CY00314K
 Shreesha Bhat is a M.S.(Pharm.) in Medicinal Chemistry from National Institute of Pharmaceutical Education and Research, India. His area of interests include chemical synthesis of biologically important molecules and developing newer methods for organic synthesis using novel catalysts.
Shreesha Bhat is a M.S.(Pharm.) in Medicinal Chemistry from National Institute of Pharmaceutical Education and Research, India. His area of interests include chemical synthesis of biologically important molecules and developing newer methods for organic synthesis using novel catalysts.