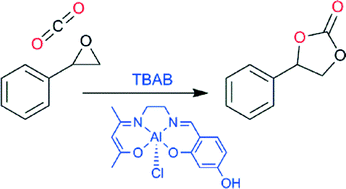
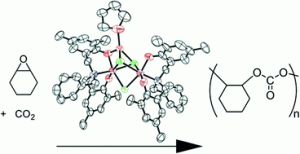
The potential of carbon dioxide to serve as a sustainable feedstock on an industrial scale is exemplified by the reaction of carbon dioxide with epoxides to form cyclic carbonates. These products possess commercial value as both solvents and electrolytes in lithium ion batteries.
In their latest Catalysis Science & Technology article, Michael North of the University of York, UK, and Antonio Otero from the Universidad de Castilla La Mancha, Spain, and colleagues investigate using bi- and trimetallic aluminium heteroscorpionate catalysts to drive this carbonate synthesis.
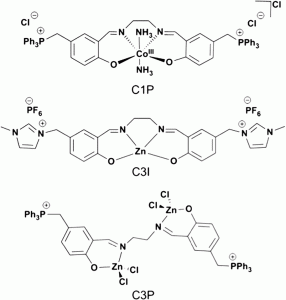
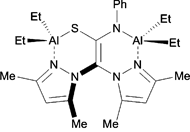 The authors subjected nineteen complexes to a screening process which involved successive elimination based on their initial reactivity towards styrene oxide. The catalysts differed in their nuclearities and included either alkyl or phenoxide ligands, in addition to having one or more bis-pyrazole ligands. They found that the bi- and trinuclear catalysts, in the presence of a tetrabutylammonium bromide co-catalyst, exhibited the highest conversions of monomer at 10 bar pressure and room temperature; thus, the authors subsequently tested these six complexes at 1 bar pressure. Among these, a trimetallic, alkyl aluminium complex gave complete conversion to styrene carbonate and was subjected to further optimization studies.
The authors subjected nineteen complexes to a screening process which involved successive elimination based on their initial reactivity towards styrene oxide. The catalysts differed in their nuclearities and included either alkyl or phenoxide ligands, in addition to having one or more bis-pyrazole ligands. They found that the bi- and trinuclear catalysts, in the presence of a tetrabutylammonium bromide co-catalyst, exhibited the highest conversions of monomer at 10 bar pressure and room temperature; thus, the authors subsequently tested these six complexes at 1 bar pressure. Among these, a trimetallic, alkyl aluminium complex gave complete conversion to styrene carbonate and was subjected to further optimization studies.
The team of researchers also studied the effect of water on the reaction to elucidate the catalytically active species. They discovered that a small amount of water (0.75 mol % or less) produced no effect, pointing towards the presence of a partially hydrolyzed, oligomeric structure containing bridging aluminium units. Although ineffective for the transformation of more challenging internal epoxides, the optimized catalyst proved to be highly efficient towards a variety of terminal epoxides. By performing mechanistic studies, it appeared that the reaction follows first order kinetics, implying that cooperative catalysis between aluminium ions does not occur.
This synergistic catalytic system, comprised of equimolar amounts of a trimetallic aluminium complex and tetrabutylammonium bromide, was determined to be the third most active catalyst for the synthesis of cyclic carbonates from terminal epoxides under ambient conditions.
Read this Hot article now:
Synthesis of cyclic carbonates catalysed by aluminium heteroscorpionate complexes
José A. Castro-Osma, Carlos Alonso-Moreno, Agustín Lara-Sánchez, Javier Martinez, Michael North, and Antonio Otero
Catal. Sci. Technol., 2014, DOI: 10.1039/C3CY00810J
This article is also part of the upcoming themed issue Catalytic Conversion and Use of Carbon Dioxide for Value-Added Organics – to be published Spring 2014.
 |
Jenna Flogeras obtained her M.Sc. in Chemistry from the University of New Brunswick (Fredericton), Canada. She is currently working towards her Ph.D. at Memorial University of Newfoundland, under the supervision of Dr Francesca Kerton. Her research is focused on the synthesis of biodegradable polymers using main-group metal complexes as catalysts. |
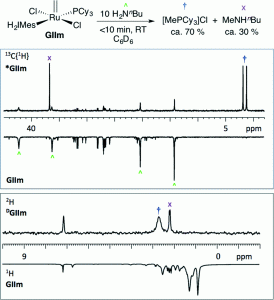 D. Fogg et al. have discovered a powerful means to observe the behaviour of Grubbs metathesis catalysts.
D. Fogg et al. have discovered a powerful means to observe the behaviour of Grubbs metathesis catalysts.