Recovering homogeneous catalysts at the end of a chemical reaction can be tricky as the catalysts are in the same phase as the products. But scientists in Canada have now found a way to do this that doesn’t suffer from the slow reaction rates that affect current catalyst recovery systems. Currently, catalyst recovery systems in use in industry rely on an aqueous/organic mixture. The catalyst is dissolved in the aqueous phase and the reagents are in the organic phase. The problem with this, though, is that because the catalyst and reagents only meet at the interface between the two, the reaction is slow.
Philip Jessop and colleagues at Queen’s University, Ontario, have come up with a solvent system that switches from a single phase for a quick reaction to two phases for quick and easy separation. The team tested their system on a homogeneous catalytic reaction. First, they carried out the reaction in a one-phase switchable water/organic solvent mix and then switched the water’s properties to get two phases – one holding the product and the other holding the catalyst. ‘Switchable water is a CO2-switchable solvent – its physical properties can be changed by applying or removing CO2, ‘ explains Sean Mercer from Jessop’s group.
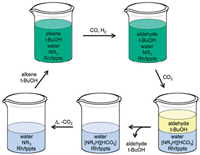
Monophasic hydroformylation and biphasic separation in a liquid mixture of switchable water and tert-butanol
The switchable water solvent mix comprises water and a tertiary amine base, resulting in water free from salts. Introducing CO2 leads to the formation of salts (carbonic acid forms in the water and protonates the amine base, generating charged species or salts), forcing out the organic solvent. The product, which is in the organic solvent, can then be removed, leaving behind the catalyst, which stays in the water. Removing the CO2 by heating and flushing with air causes the charged species to revert back to their original uncharged form, making the water salt-free once more. ‘Fresh reagents and organic solvent can then be added and the reaction can be run again and again,’ says Mercer.
‘Others have done this in a slightly different way, in which the originally hydrophobic catalyst switches into water on passing CO2, but Jessop’s “switchable water” approach has the advantage that he can use simple water soluble ligands that can be bought off the shelf, whilst the ligand switching requires specially designed ligands that are difficult to make,’ says David Cole-Hamilton, an expert in homogeneous catalysis at the University of St Andrews, UK. However, he does point out that there are still problems to be addressed, including a fall off in conversion after several cycles, which he says can almost certainly be fixed by improved reactor and recycler design and by the rigorous exclusion of air.
Another issue, adds Mercer, is that they perform the catalysis in a highly basic medium, so certain reactions can’t be performed. ‘We also need to enlarge the number of reactions that can be performed using this solvent system, as we only demonstrated the hydroformylation of alkenes to aldehydes,’ he says. ‘A second issue is we sometimes observe slight leaching of our precious metal catalyst into the organic phase so it is lost from the process. In the immediate future, we need to find catalysts that leach less, or move to less expensive metals so that losses aren’t as detrimental monetarily.’
Written by Elinor Richards
Recycling of a homogeneous catalyst using switchable water
Sean M. Mercer, Tobias Robert, Daniel V. Dixon and Philip G. Jessop
DOI: 10.1039/C2CY20095C
 Catalysts are key to some of the most important reactions on the planet; a world without the Haber process or catalysts to crack crude oil is difficult to imagine. Not to mention the enzymatic reactions that are crucial to all life on earth.
Catalysts are key to some of the most important reactions on the planet; a world without the Haber process or catalysts to crack crude oil is difficult to imagine. Not to mention the enzymatic reactions that are crucial to all life on earth.