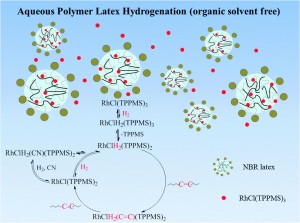
Hydrogenated acrylonitrile butadiene rubber (HNBR) is an important elastomer heavily relied on by the automotive and petroleum industry. Identified for its tensile strength and ability to resist oxidative degradation, HNBR is used to make seals, hoses and belts. On an industrial scale, HNBR is synthesized by the hydrogenation of unsaturated acrylonitrile butadiene rubber (NBR). This process involves an organic solvent, hydrogen gas and a transition metal catalyst.
In this advance article, Rempel, Pan and co-workers have developed a green method to hydrogenate NBR that employs water-soluble Rhodium catalysts in purely aqueous media. Rhodium chloride monosulfonated triphenylphosphine (RhCl(TPPMS)3, 0.52 mmol L-1) catalysed the hydrogenation of NBR (50 g L-1) with a 95% conversion to HNBR in 9 hours at 1000 psi and 100 °C. The solubility of the Rhodium catalyst was critical to the success of the reaction, creating an effective relative partition between the water and polymer phases. The authors also found that NBR starting materials containing gel resulted in lower conversions because this structure limits contact between the active catalyst and substrate.
To read more, follow the link below:
Hydrogenation of acrylonitrile-butadiene copolymer latex using water-soluble rhodium catalysts
Yin Liu, Hanmiroo Kim, Qinmin Pan, and Garry L. Rempel
Catal. Sci. Technol., 2013, Advance Article, DOI: 10.1039/c3cy00257h
 Tien Nguyen is a web contributor working towards her PhD in David Nicewicz’s research group at the University of North Carolina at Chapel Hill, USA. Her current area of research focuses on anti-Markovnikov hydroamination of alkenes using photoredox catalysis.
Tien Nguyen is a web contributor working towards her PhD in David Nicewicz’s research group at the University of North Carolina at Chapel Hill, USA. Her current area of research focuses on anti-Markovnikov hydroamination of alkenes using photoredox catalysis.