The latest themed issue from Catalysis Science and Technology, “Catalysis on Chiral Surfaces: From Fundamental Aspects to Application”, contains a plethora of articles reporting on the latest heterogeneous catalysts with significant stereospecific properties for asymmetric or chiral transformations.
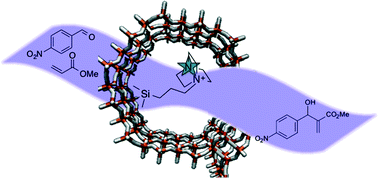
In an article from the group of Dr Raja at the University of Southampton, UK, S. Newland et al. demonstrate the importance of anchoring organocatalysts to the inner walls of mesoporous silica to attain highly active and selective catalysts. Traditionally, organocatalysts are used in a homogeneous fashion, however this often leads to labour and energy intensive recollection for recycling of the catalyst. The covalent anchoring of cinchonine to the inner walls of mesoporous silicas not only gains the facile recycle benefits of a heterogeneous catalyst, but can increase the activity 6-fold for Michael addition reactions compared with the homogeneous analogue. And this is all achieved without sacrificing the enantioselectivity of the catalyst!
The anchoring methods were applied to DABCO, another molecule used as a chemoselective organocatalyst to show that the method could be applied to a variety of different catalysts with equally impressive results. It is easy to envisage these anchoring methods being significantly useful to any researchers working in the area of chiral organocatalysis.
Want to find out more on the synthesis and origin of these catalytic properties? Read the full article now!
Highly effective design strategy for the heterogenisation of chemo- and enantioselective organocatalysts
Stephanie H. Newland, David J. Xuereb, Enrica Gianotti, Leonardo Marchese, Ramon Rios and Robert Raja
Catal. Sci. Technol., 2015,5, 660-665, DOI: 10.1039/C4CY00895B,