In this recent communication, W. Zhang and co-workers have demonstrated a novel way to form covalent porphyrinic frameworks (CPFs) using squaraine and hydrazine linkers. By using purely organic moieties the researchers have eliminated the need for metal nodes that are present in the more traditional porous coordination polymers.
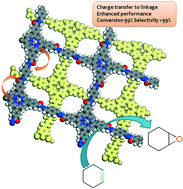
Using the principles of supramolecular chemistry the authors have used a metallation process to introduce manganese, a well-known and abundant redox active transition metal, into the cavity of the porphyrin component of the framework. Thus, an easily accessible active site for redox catalysis was generated and subsequently demonstrated to be active for the selective oxidation of aliphatic molecules.
Interestingly, it was also shown that the choice of linker can have an effect on the resulting catalytic activity of these new materials. Preliminary characterisation showed a correlation between the extended conjugation of the organic framework, the state of the manganese and the activity/selectivity of the catalytic processes. This phenomenon, often observed in homogeneous porphyrin catalysts, has been elegantly incorporated into these heterogeneous analogues. One can hypothesise that in future, by careful design of the organic linkers joining the porphyrin units, it may be possible to fine-tune the material for different catalytic reactions.
To find out more, why not take a look at the article now?
Bottom-up approach to engineer two covalent porphyrinic frameworks as effective catalysts for selective oxidation
Weijie Zhang, Pingping Jiang, Ying Wang, Jian Zhang and Pingbo Zhang
Catal. Sci. Technol., 2015, DOI: 10.1039/C4CY00969J