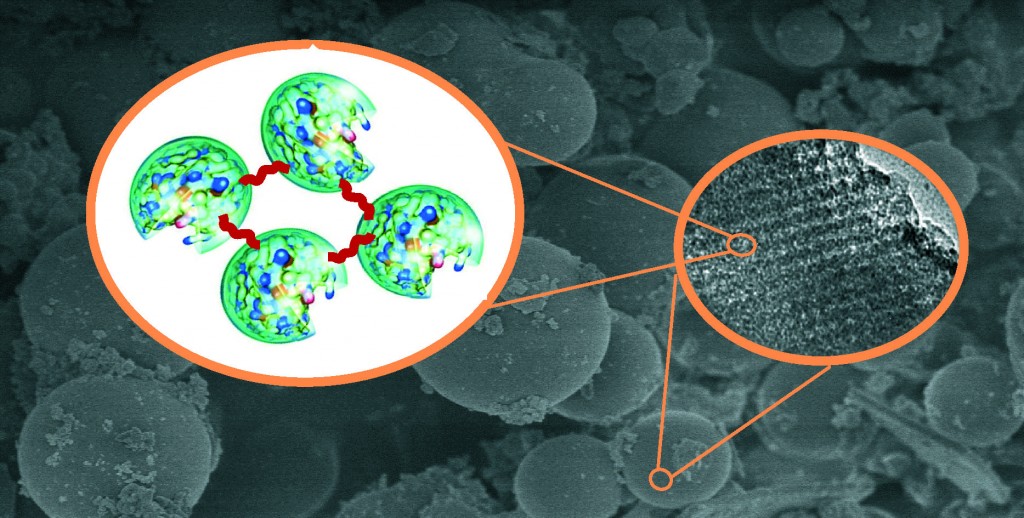
A themed issue on Enzyme Immobilization was recently published in Chemical Society Reviews encompassing the advances made in the field of enzyme immobilization and its importance in the Industrial arena. The credit goes to a simple discovery by Nelson and Griffin, who in 1916 rediscovered that artificial carrier-bound invertase on Al(OH)3 and charcoal was still catalytically active. They put to rest the claims that substances like charcoal caused inhibition of the enzymes (due to adsorption) and established that adsorption had no role in the decreased activity of the enzymes. This discovery laid the foundation for the immobilized enzymes to find wide applications in the chemical industry.
The carriers used for immobilization include natural and synthetic polymers like cellulose, starch, polystyrene, sephadex and inorganic carriers like clay, kaolinite, silica gel etc. Of these, Mesoporous silica materials (MPS) have been found to be an attractive alternative due to their intrinsic properties. The immobilization of the enzymes on these carriers are generally carried out by physical adsorption or covalent binding, but face the problem of enzyme leaching. In order to overcome this problem, the Cross-linked enzyme aggregates (CLEAs) method has emerged of late and has been successful to a certain extent. In the present paper, the authors have explored the CLEAs of lipase from Candida sp. 99-125 immobilized in MPS and found them to be thermally and catalytically stable with improved enzymatic activities.
As a measure of their improved properties, the activity and stability of the Cross-linked lipases in MPS (nicknamed CLL@MPA) were compared with the simple adsorbed lipases (ADL@MPA) and the native enzymes, and were found to be highly stable (at high temperatures as well as on shaking) with improved hydrolytic, esterification and transesterification activites. Although these lipases (from candida sp. 99-125) were less active than the commercially available Novzyme 435 (from candida antarctica), their cheaper costs make them a promising alternative for industrial applications.
This study thus paves the way for cheaper and effective enzyme immobilization options, which can be further extended to other enzymes and lead to potential advances in various enzyme-based industrial processes.
Read more at:
Formation of lipase Candida sp. 99-125 CLEAs in mesoporous silica: characterization and catalytic properties
Jing Gao, Lianlian Shi, Yanjun Jiang, Liya Zhou and Ying He
Catal. Sci. Technol., 2013, Accepted Manuscript
DOI: 10.1039/C3CY00412K
 Shreesha Bhat is a M.S.(Pharm.) in Medicinal Chemistry from National Institute of Pharmaceutical Education and Research, India. His area of interests include chemical synthesis of biologically important molecules and developing newer methods for organic synthesis using novel catalysts.
Shreesha Bhat is a M.S.(Pharm.) in Medicinal Chemistry from National Institute of Pharmaceutical Education and Research, India. His area of interests include chemical synthesis of biologically important molecules and developing newer methods for organic synthesis using novel catalysts.