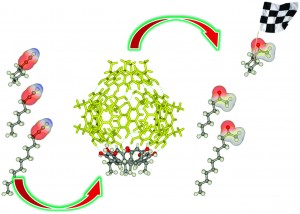
Synthetic chemists have long been attempting to attain the exquisite levels of substrate selectivity offered by enzymes. Heterogeneous catalysts can provide high selectivities through the control of their porosity. While there are some strategies for achieving selectivity with homogeneous catalysis, this field still lags behind its enzymatic and heterogeneous counterparts.
In this advance article, Strukul and co-workers demonstrated substrate selectivity in the hydration of alkynes using a NHC-Au(I) catalyst encapsulated in a hexameric resorcin host. In the presence of the encapsulated catalyst the cyclic aliphatic alkyne was converted to product faster than the longer chain linear substrates. The authors ascribe this effect to the better fit of the cyclic substrate into the host cavity. Aromatic substrates were also tested and showed low yields likely due to their increased rigidity. Overall, aliphatic and aromatic alkynes were hydrated in low to modest yields but the observed trends serve as a valuable proof of concept.
To read more, click the link below:
Substrate selectivity in the alkyne hydration mediated by NHC-Au(I) controlled by encapsulation of the catalyst within a hydrogen bonded hexameric host
Alessandra Cavarzan, Joost N. H. Reek, Francesco Trentin, Alessandro Scarso, and Giorgio Strukul
Catal. Sci. Technol. 2013, Advance Article, DOI: 10.1039/c3cy00300k
 Tien Nguyen is working towards her PhD in David Nicewicz’s research group at the University of North Carolina at Chapel Hill, USA. Her current area of research focuses on anti-Markovnikov hydroamination of alkenes using photoredox catalysis.
Tien Nguyen is working towards her PhD in David Nicewicz’s research group at the University of North Carolina at Chapel Hill, USA. Her current area of research focuses on anti-Markovnikov hydroamination of alkenes using photoredox catalysis.