A study published in the journal Diabetes Care estimated that in 2000, 171 million people worldwide had diabetes. People with diabetes have high blood sugar levels so biosensors that detect glucose are crucial in the diagnosis and treatment of this disease. Many diagnostic glucose sensors have been developed with widespread use in clinical and biotechnology applications.
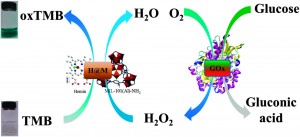
In this Catalysis Science & Technology advance article, Liu and co-workers developed a colorimetric method for the detection of H2O2. Hydrogen peroxide is a by-product formed when glucose is oxidized by glucose oxidase (GOx), so coupling these events is a common strategy for glucose detection. The resultant H2O2 in the presence of an oxidation catalyst oxidizes 3,3,5,5-tetramethylbenzidine (TMB) to the diimine (oxTMB) producing a deep blue color. The researchers synthesized a composite material (H@M)to catalyse the oxidation by anchoring Hemin, to an amino-containing MOF (MIL-101(Al)-NH2).
This represents the first use of a MOF-artificial enzyme in glucose detection. Immobilizing Hemin on a metal-organic framework prevents problematic oxidative degradation and molecular aggregation. Also, the pore structure of the MOF mimics protein structure, which is important for activity and selectivity. The catalytic activity of this enzyme mimic is dependent on pH, temperature and H2O2 concentration. However, glucose oxidation is optimal at a pH of 7.0 and TMB oxidation by H@M is optimal at a pH of 5.0. The glucose detection is finally realized by first reacting glucose with GOx then adding a TMB and H@M solution and adjusting the acidity of the solution.
To read more, follow the link below:
Hemin@metal-organic framework with peroxidase-like activity and its application to glucose detection
Feng-Xiang Qin, Shao-Yi Jia, Fei-Fei Wang, Song-Hai Wu, Jia Song and Yong Liu
Catal. Sci. Technol. 2013, Advance Article, DOI: 10.1039/c3cy00268c
 Tien Nguyen is a web contributor working towards her PhD in David Nicewicz’s research group at the University of North Carolina at Chapel Hill, USA. Her current area of research focuses on anti-Markovnikov hydroamination of alkenes using photoredox catalysis.
Tien Nguyen is a web contributor working towards her PhD in David Nicewicz’s research group at the University of North Carolina at Chapel Hill, USA. Her current area of research focuses on anti-Markovnikov hydroamination of alkenes using photoredox catalysis.