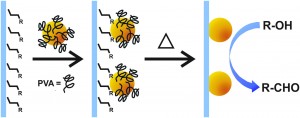
Aldehydes are valuable synthetic intermediates with many methods for their preparation. But the majority of these approaches employ stoichiometric oxidants that produce toxic waste. Aerobic oxidation with molecular oxygen and a transition metal catalyst offers an environmentally benign alternative. In this advance article, Rossi and colleagues reported the first magnetically recoverable AuPd nanoparticle catalyst applied to the oxidation of primary alcohols to aldehydes.
The removal of metal catalysts supported on magnetic surfaces with an external magnetic field is an innovative and efficient method for separation. The researchers achieved linkage by dually functionalizing the support with strongly coordinating ligands and impregnating the nanoparticles with weak coordinating groups in the coordination capture method. They found that catalysts with amino-functionalized silica supports exhibited higher activity and stability to catalyst recycling than the analogous thiol supports. The authors achieved a 92% conversion of benzyl alcohol with high selectivity for benzaldehyde using 1 wt% AuPd catalyst (Fe3O4@SiO2-NH2-AuPd) under 6 bar of O2 at 100 °C. However, catalyst separation was impeded by the amino group, which had reacted with the product benzaldehyde to form an aldimine.
This issue was circumvented through the calcination of the Fe3O4@SiO2-NH2-AuPd catalyst at 500 °C for 2 hours, effectively removing the amino groups and promoting highly efficient catalyst recovery. Good yield and selectivity for the oxidation reaction was maintained and the catalyst was used in five successive reactions without loss of selectivity.
To read more, follow the link below:
Tiago A. G. Silva, Richard Landers and Liane M. Rossi
Catal. Sci. Technol., 2013, Advance Article, DOI: 10.1039/c3cy00261f
 Tien Nguyen is a web contributor working towards her PhD in David Nicewicz’s research group at the University of North Carolina at Chapel Hill, USA. Her current area of research focuses on anti-Markovnikov hydroamination of alkenes using photoredox catalysis
Tien Nguyen is a web contributor working towards her PhD in David Nicewicz’s research group at the University of North Carolina at Chapel Hill, USA. Her current area of research focuses on anti-Markovnikov hydroamination of alkenes using photoredox catalysis