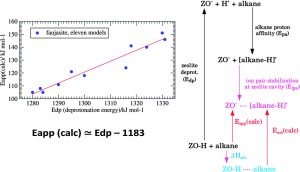
Alkane cracking is an important industrial process, and zeolites are commonly used as catalysts in the reaction. There have been many studies into the reaction, and the variation in the catalytic activity between different zeolites is often explained by the differences in the heat of alkane adsorption on the zeolite, which is determined by the pore size.
In this paper, the authors proposed that the zeolite acid strength (the BrØnsted acidity), rather than heat of alkane adsorption on the zeolite is the main factor in determining the cracking activity. They supported this view with a series of experiments and computational studies on ultra-stable Y zeolites. These include measurements of how the activation energy of cracking varies with the acidity of the zeolite, and a density functional theory calculation of the reaction. The good agreement between the model and experimental results suggests that the proposed mechanism is correct.
Find out more from their paper:
Dependence of cracking activity on the Brønsted acidity of Y zeolite: DFT study and experimental confirmation
Miki Niwa, Katsuki Suzuki, Nami Morishita, German Sastre, Kazu Okumura and Naonobu Katada
Catal. Sci. Technol., 2013, Advance Article
DOI: 10.1039/C3CY00195D, Paper